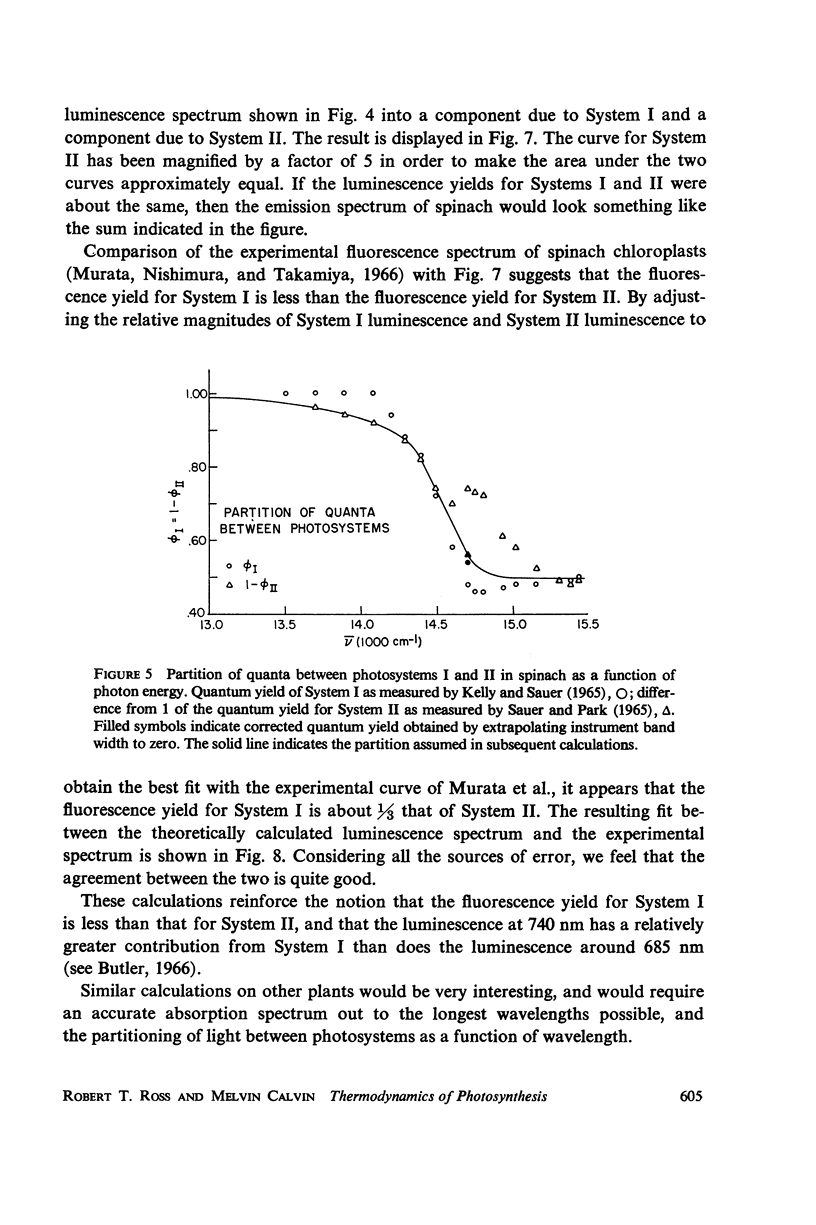
Abstract
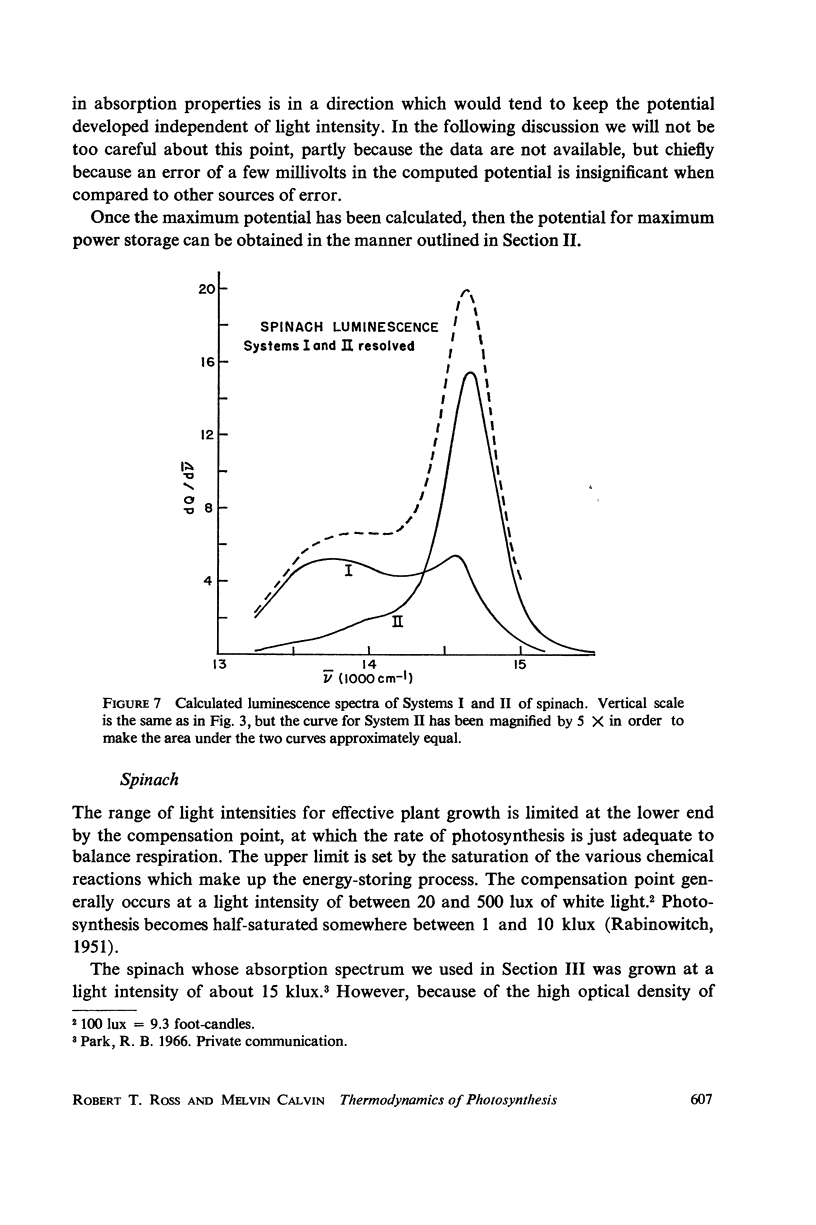
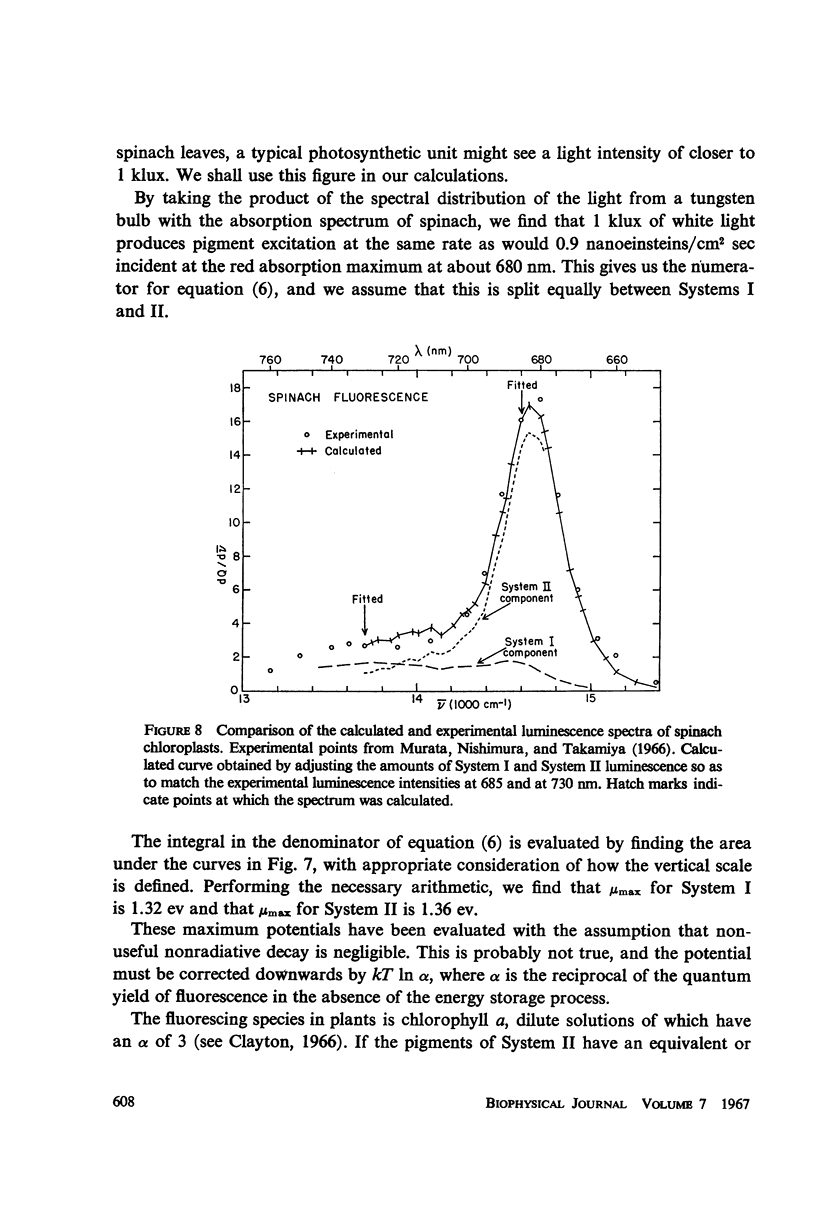
A Planck law relationship between absorption and emission spectra is used to compute the fluorescence spectra of some photosynthetic systems from their absorption spectra. Calculated luminescence spectra of purple bacteria agree well but not perfectly with published experimental spectra. Application of the Planck law relation to published activation spectra for Systems I and II of spinach chloroplasts permits independent calculation of the luminescence spectra of the two systems; if the luminescence yield of System I is taken to be one-third the yield of System II, then the combined luminescence spectrum closely fits published experimental measurement.
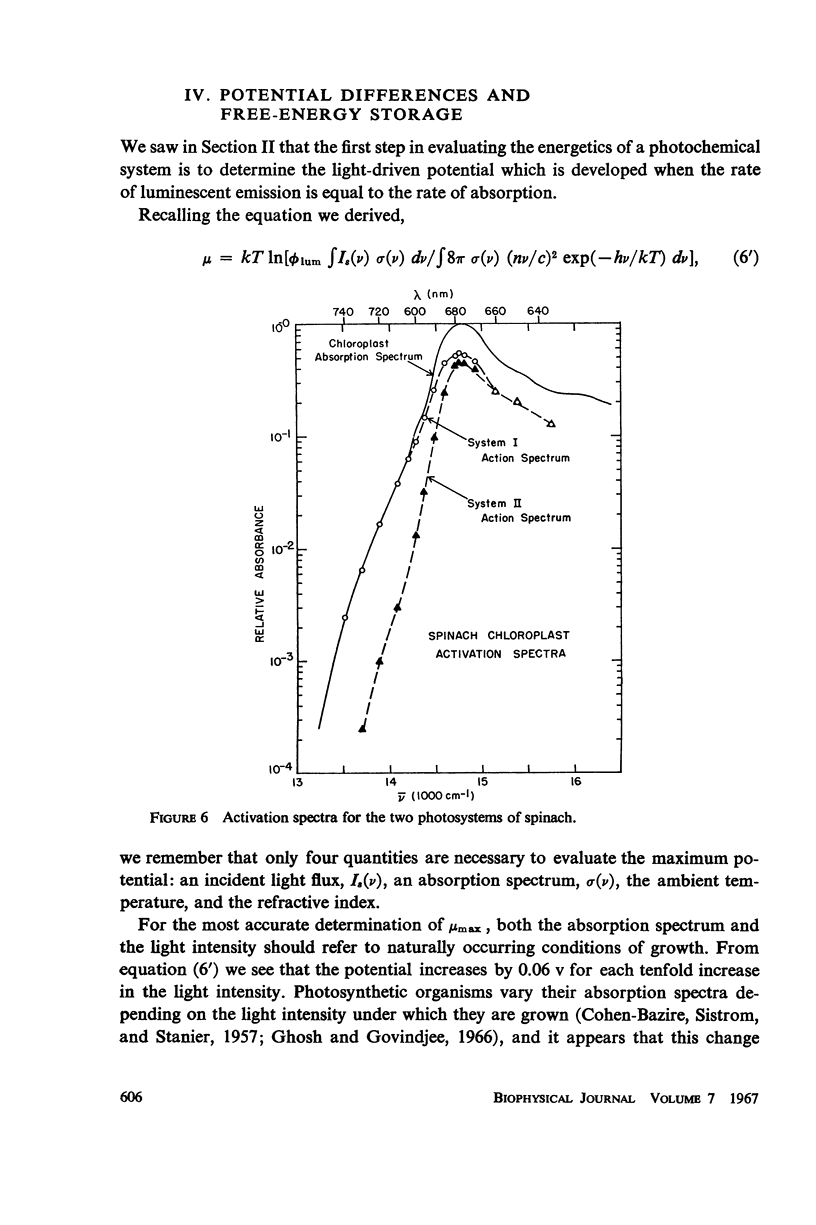
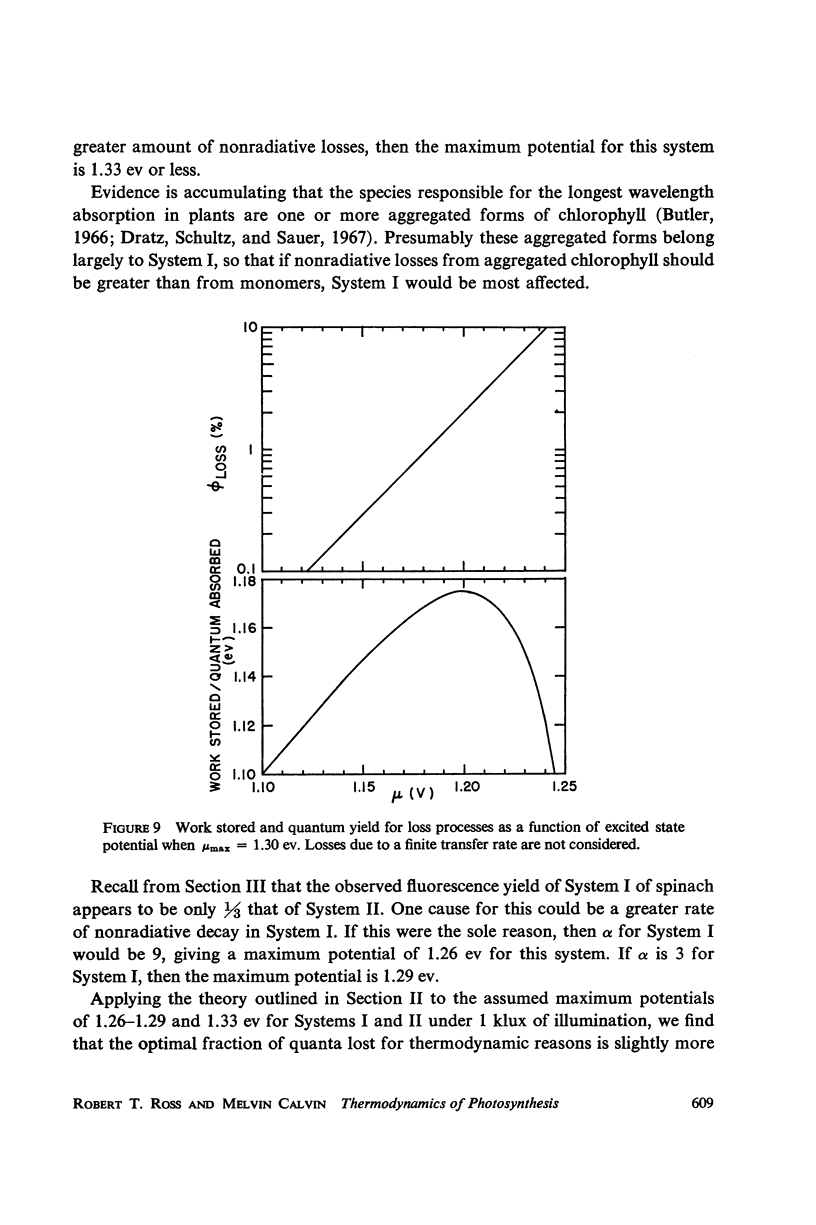
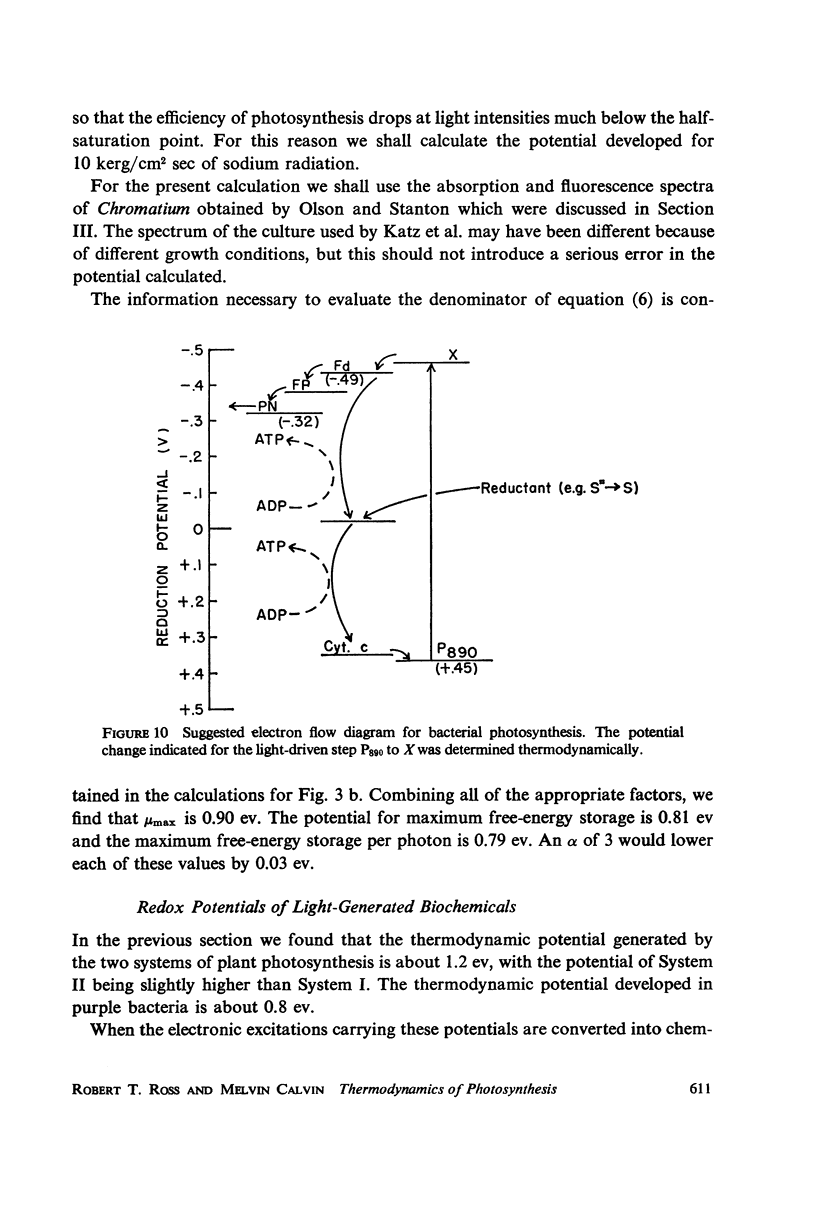
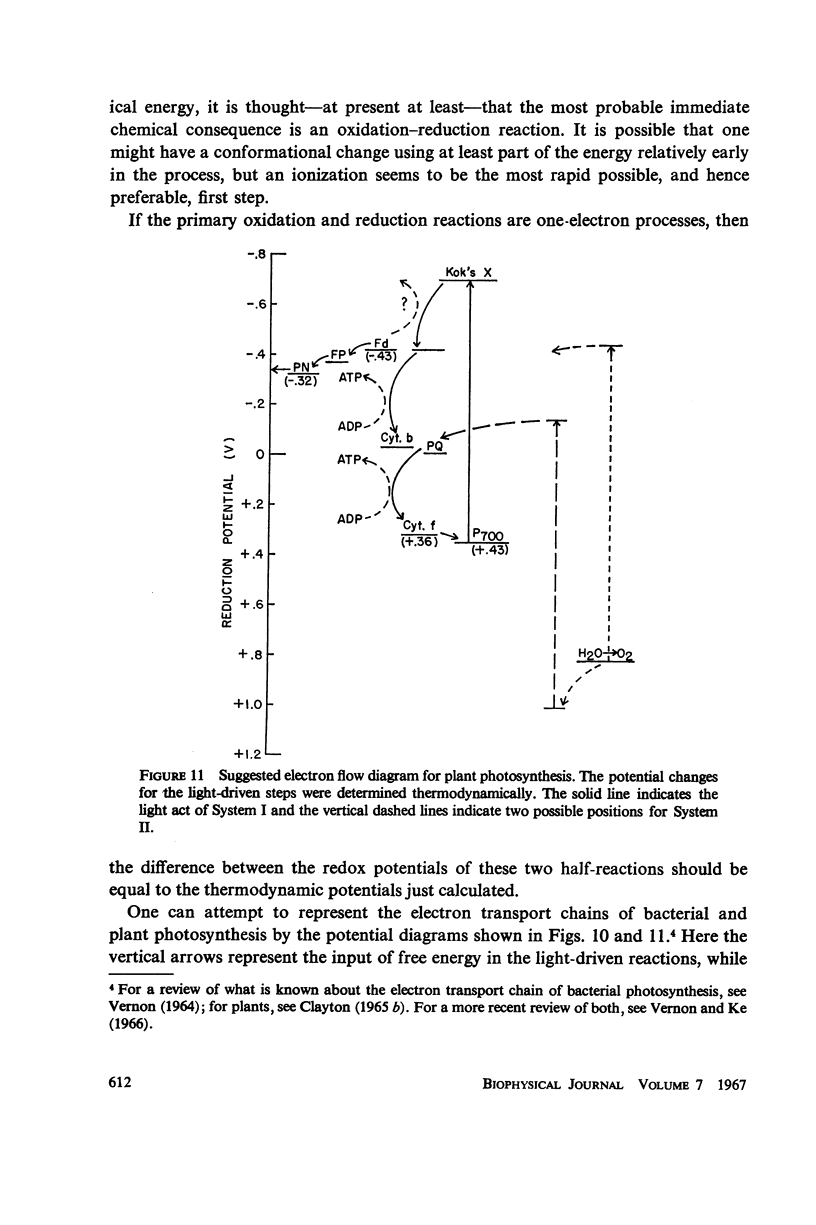
Consideration of the entropy associated with the excited state of the absorbing molecules is used to compute the oxidation-reduction potentials and maximum free-energy storage resulting from light absorption. Spinach chloroplasts under an illumination of 1 klux of white light can produce at most a potential difference of 1.32 ev for System I, and 1.36 ev for System II. In the absence of nonradiative losses, the maximum amount of free energy stored is 1.19 ev and 1.23 ev per photon absorbed for Systems I and II, respectively. The bacterium Chromatium under an illumination of 1 mw/cm2 of Na D radiation can produce at most a potential difference of 0.90 ev; the maximum amount of free energy stored is 0.79 ev per photon absorbed.
The combined effect of partial thermodynamic reversibility and a finite trapping rate on the amount of luminescence is considered briefly.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertsch W., Azzi J. R., Davidson J. B. Delayed light studies on photosynthetic energy conversion. I. Identification of the oxygen-evolving photoreaction as the delayed light emitter in mutants of Scenedesmus obliquus. Biochim Biophys Acta. 1967 Jul 5;143(1):129–143. doi: 10.1016/0005-2728(67)90116-8. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. CHARACTERISTICS OF FLUORESCENCE AND DELAYED LIGHT EMISSION FROM GREEN PHOTOSYNTHETIC BACTERIA AND ALGAE. J Gen Physiol. 1965 Mar;48:633–646. doi: 10.1085/jgp.48.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratz E. A., Schultz A. J., Sauer K. Chlorophyll-chlorophyll interactions. Brookhaven Symp Biol. 1966;19:303–318. [PubMed] [Google Scholar]
- Ghosh A. K., Govindjee Transfer of the excitation energy in Anacystis nidulans grown to obtain different pigment ratios. Biophys J. 1966 Sep;6(5):611–619. doi: 10.1016/S0006-3495(66)86681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J., Sauer K. Action spectrum and quantum requirements for the photoreduction of cytochrome c with spinach chloroplasts. Biochemistry. 1965 Dec;4(12):2798–2802. doi: 10.1021/bi00888a033. [DOI] [PubMed] [Google Scholar]
- Mayne B. C., Clayton R. K. Luminescence of chlorophyll in spinach chloroplasts induced by acid-base transition. Proc Natl Acad Sci U S A. 1966 Mar;55(3):494–497. doi: 10.1073/pnas.55.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Nishimura M., Takamiya A. Fluorescence of chlorophyll in photosynthetic systems. I. Analysis of "weak light effect" in isolated chloroplasts. Biochim Biophys Acta. 1966 Feb 7;112(2):213–222. doi: 10.1016/0926-6585(66)90322-0. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., ARNOLD W. Light production by green plants. J Gen Physiol. 1951 Jul;34(6):809–820. doi: 10.1085/jgp.34.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K., Biggins J. Action spectra and quantum yields for nicotinamide--adenine dinucleotide phosphate reduction by chloroplasts. Biochim Biophys Acta. 1965 May 25;102(1):55–72. doi: 10.1016/0926-6585(65)90202-5. [DOI] [PubMed] [Google Scholar]
- Sauer K., Park R. B. The Hill reaction of chloroplasts. Action spectra and quantum requirements. Biochemistry. 1965 Dec;4(12):2791–2798. doi: 10.1021/bi00888a032. [DOI] [PubMed] [Google Scholar]
- Szalay L., Rabinowitch E., Murty N. R., Govindjee Relationship between the Absorption and Emission Spectra and the "Red Drop" in the Action Spectra of Fluorescence In Vivo. Biophys J. 1967 Mar;7(2):137–149. doi: 10.1016/S0006-3495(67)86580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


