Abstract
RNA-1 of Peanut clump virus (PCV) encodes the proteins P131 and P191, containing the signature motifs of replication proteins, and P15, which regulates viral RNA accumulation. In PCV-infected protoplasts both P131 and P191 were immunodetected in the perinuclear region. Laser scanning confocal microscopy (LSCM) showed that P131 and P191 colocalized with neosynthesized 5-bromouridine 5′-triphosphate-labeled RNA and double-stranded RNA, demonstrating that they belong to the replication complex. On the contrary, the P15 fused to the enhanced green fluorescent protein (EGFP) never colocalized with the two proteins. In endoplasmic reticulum (ER)-GFP transgenic BY-2 protoplasts, the distribution of the green fluorescent-labeled ER was strongly modified by PCV infection. LSCM showed that both P131 and P191 colocalized with ER green fluorescent bodies accumulating around the nucleus during infection. The replication process was not inhibited by cerulenin and brefeldin A, suggesting that PCV replication does not depend on de novo-synthesized membrane and does not require transport through the Golgi apparatus. Electron microscopy of ultrathin sections of infected protoplasts showed aggregates of broken ER but also visualized vesicles, some of which resembled modified peroxisomes. The results suggest that accumulation of PCV during infection is accompanied by specific association of PCV RNA-1-encoded proteins with membranes of the ER and other organelles. The concomitant extensive rearrangement of these membranous structures leads to the formation of intracellular compartments in which synthesis and accumulation of the viral RNA occur in defined areas.
Replication is a key event of the viral multiplication cycle that ultimately determines the success of the viral infection. For positive-stranded viruses, replication is thought to occur in complexes containing viral RNA template, viral RNA-dependent RNA polymerase (RdRp), cellular factors, and, in some cases, virus-encoded accessory proteins (27, 57). All results obtained so far show that RNA replication occurs in close association with intracellular membranes, which generally undergo extensive reorganization in the virus-infected cell (9).
Depending upon the virus, the membrane component of the replication complex may be of different origins: membranes of the endoplasmic reticulum (ER) are implicated in viral replication of picorna-like viruses such as comoviruses (11) and potyviruses (50) and of alpha-like viruses such as tobamoviruses (42) and bromoviruses (44, 45). Cytoplasmic invaginations of chloroplast membranes are associated with Tymovirus replication complexes (18, 41), and multivesicular bodies derived from peroxisomes, mitochondria, or vacuoles are the sites of replication for other viruses: cucumber mosaic and tomato aspermy viruses (21), Tomato bushy stunt virus (52), Cymbidium ringspot virus and Carnation Italian ringspot virus (47), and Alfalfa mosaic virus (AMV) (55).
In animal cells, the replication of viruses of the Togaviridae and Coronaviridae families occurs in modified endosomes or lysosomes (17, 30, 40, 56). Flavivirus RNA synthesis is thought to occur in vesicle packets derived from the trans-Golgi membranes (29, 58), whereas membranes of the ER, of the Golgi apparatus, and of lysosomes have been detected in the vesicles generated by poliovirus replication (51).
In a number of cases (e.g., poliovirus, alphaviruses, and Brome mosaic virus), all of the viral replication proteins are localized within the replication complex (2, 3, 5, 6, 17, 39, 45). On the other hand, for Flavivirus Kunjin, the replicative proteins and RNA viral synthesis sites colocalize in vesicle packets, while other nonstructural proteins are associated with modified membranes from the intermediate compartment (29). For Tobacco etch virus, only the 6-kDa protein and the viral RNA replication complex were associated with vesicles derived from the ER, whereas NIa and NIb, which are also required for replication, predominantly accumulated in the nucleus (43, 50).
To gain insight into the interactions between viral and host factors during Peanut clump virus (PCV) RNA replication, we have investigated the in situ localization of the replicase proteins and the RNA synthesis sites. PCV, a type member of the Pecluvirus genus, is a positive-strand RNA virus of the alphavirus-like family. The PCV genome is composed of two molecules of RNA. RNA-1 is able to replicate independently of RNA-2 in protoplasts, but both RNAs are indispensable for plant infection (25). Two N-terminally overlapping proteins encoded by RNA-1 (P131 and P191) are essential replication proteins. P131 contains sequence motifs common to methyltransferases and helicases, while P191 contains the signature motifs of RNA-dependent RNA polymerases in its C-terminal extension (20, 24). The RNA-1-encoded protein P15, on the other hand, is not an essential replication factor but is required for efficient viral RNA accumulation. This protein was not detected near the sites of viral RNA synthesis (15) and therefore is probably not a component of the replication complex. In the present study, we have further investigated the localization of the viral RNA replication complex and provide evidence that both P131 and P191 are present at the replication sites. Using protoplasts from transgenic lines of tobacco BY-2 cells, we demonstrate that the ER, but not the Golgi apparatus, undergoes extensive reorganization upon PCV infection, and we show that the viral RNA replication complex is associated with the modified ER membranes.
MATERIALS AND METHODS
Plasmids and transcripts.
Viral RNA was extracted from purified PCV (isolate PCV2) (31). Plasmids pPC1 and pPC2 are full-length cDNA clones of PCV RNA-1 and RNA-2, respectively, used to produce infectious transcripts T1 and T2 corresponding to RNA-1 and RNA-2 (25). pRep-EG15 is a chimeric RNA molecule consisting of the 5′-terminal sequence of RNA-2, including the CP gene, and the 3′-terminal sequence of RNA-1, including the P15 gene fused to enhanced green fluorescent protein (EGFP). It has been previously shown that this RNA coreplicates with RNA-1 and RNA-2 (15). To construct pRep-RFP15, the fragment NcoI-EcoRI of pRep-EG15 corresponding to the EGFP gene was replaced by the NcoI-NotI fragment excised from pDsRed1-N1 (Clontech), corresponding to the Discosoma red fluorescent protein (RFP) cistron. Capped in vitro transcripts were obtained with a Ribomax transcription kit (Promega) according to the manufacturer’s instructions after the plasmids had been linearized with MluI for pPC1 or HindIII for pPC2.
Inoculation of protoplasts and plants.
Protoplasts from BY-2 tobacco cells (35) were prepared as previously described (25). ER-GFP protoplasts were obtained from a transgenic ER-GFP BY-2 cell line which constitutively expresses GFP fused to the C-terminal HDEL ER retention signal (gift from K. Danna, University of Colorado, Boulder). Man1::GFP protoplasts were obtained from a transgenic Man1::GFP cell line expressing GFP fused to the α-1,2 mannosidase I, a cis-Golgi resident protein (a gift from L.A. Staehelin [described in reference 36]). Inoculation of protoplasts (106 in 0.5 ml) was done by electroporation as described previously (15) with 5 μg of infectious transcripts or 2.5 μg of viral RNA.
ER-GFP Nicotiana benthamiana plants expressing the GFP targeted to the lumen of the ER (a gift from D. Baulcombe, John Innes Center, Norwich, United Kingdom) were inoculated with purified PCV RNA (5 μg per leaf) and grown at 26°C with a photoperiod of 16 h of light and 8 h of dark.
Antibodies.
Peptides corresponding to the 15 C-terminal amino acids of P131 and to the 15 C-terminal amino acids of P191 were synthesized and coupled with ovalbumin. About 200 μg of each purified peptide was injected into a rabbit at 2-week intervals. The serum was collected after the third boost. Antibodies were purified from serum by affinity chromatography with the peptide used to raise the serum linked to CNBr-activated Sepharose 4B (Pharmacia Biotech, Uppsala, Sweden). Chromatography was performed according to the manufacturer’s protocol.
In vitro translation and Western blots.
In vitro translation with wheat germ extract was done as previously described (23) with [35S]methionine in the incubation medium. Proteins extracted from infected plants or obtained by in vitro synthesis were separated by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis and then electrotransferred for 2 h at 0.8 mA/cm2 onto Immobilon-P membranes (Millipore). 35S-labeled proteins were revealed by autoradiography. The membranes containing viral proteins synthesized in vivo were incubated with 5% powdered milk-1% Tween 20 in//// phosphate-buffered saline (PBS) for 2 h and then overnight with P131 or P191 antiserum. The membranes were washed in 1% Tween 20 in PBS and incubated for 2 h with peroxidase-coupled anti-rabbit immunoglobulin G in 5% milk-1% Tween 20 in PBS. After a wash with 0.5% Tween 20 in PBS, bound antibodies were detected by ECL chemiluminescent reaction (Pierce) according to the manufacturer’s instructions.
Northern blots.
Total RNA was extracted from protoplasts as previously described (15), and viral RNAs were detected by hybridization with a specific in vitro-transcribed 32P-labeled RNA probe that corresponds to the minus strand of the 3′-terminal 124 nucleotides, which is common to both genomic RNAs (33). Radioactive signals were detected by autoradiography.
Immunofluorescent detection of viral proteins.
At 24 and 48 h postinfection (hpi), harvested protoplasts were transferred to a fixing solution composed of 1% glutaraldehyde in BY-2 tobacco cell culture medium (MS), washed twice in PBS and once in 0.1% NaBH4-PBS, and stored at 4°C. After incubation for 1 h in the blocking solution (5% bovine serum albumin [BSA], 5% normal goat serum, and 0.1% cold water fish skin gelatin in PBS), protoplasts were incubated overnight with the primary P131 or P191 antibodies. After six washes with 0.1% BSA-c (Aurion, Wageningen, The Netherlands) in PBS, protoplasts were further incubated with Alexa 488, 568, or 633 goat anti-rabbit antibodies (Molecular Probes) for 4 h. After six washes in 0.1% BSA-c in PBS, the protoplasts were observed with a Zeiss LSM 510 confocal microscope.
In vivo RNA and dsRNA labeling.
At 24 hpi, protoplasts were incubated for 1 h with 10 μg of actinomycin D/ml and then for 6 h with 100 μM 5-bromouridine 5′-triphosphate (BrUTP; Sigma). Incorporation was stopped by addition of the fixation medium (1% glutaraldehyde in MS) with gentle agitation for 30 min. The protoplasts were then processed as for immunofluorescent detection of proteins by using mouse anti-BrUTP primary antibody (Sigma) and Alexa 488- or 568-conjugated secondary antibody to detect BrUTP incorporation or by using guinea pig polyclonal antibody to detect double-stranded RNA (dsRNA; gift from J. Y. Lee, Macfarlane Burnet Center for Medical Research, Fairfield, Australia) and Alexa 568 secondary antibody to detect dsRNA.
Electron microscopy.
For transmission electron microscopy, cells were fixed for 1 h with 1% glutaraldehyde in MS and immersed for 15 min at room temperature in 1 ml of a primary fixative containing 2% (vol/vol) glutaraldehyde and 0.1 ml of saturated picric acid in 25 mM potassium phosphate (pH 7.4), before incubation at 4°C for 16 h. After four washes in 25 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 7.0] at room temperature, the cells were transferred to a secondary fixative containing 2% (wt/vol) osmium tetroxide and 0.5% (wt/vol) potassium ferrocyanide in 25 mM PIPES (pH 7) for 2 h at room temperature. The cells were then washed twice in 25 mM PIPES (pH 7) and twice in distilled water before transfer to 2% (wt/vol) aqueous uranyl acetate for 16 h at 4°C. After two washes in water, the cells were dehydrated in an acetone series and embedded in Spurr’s resin. Sections were double stained with uranyl acetate and lead citrate before being examined with an electron microscope operating at 80 kV (Hitashi H600).
RESULTS
Detection of the 131K and 191K replication proteins.
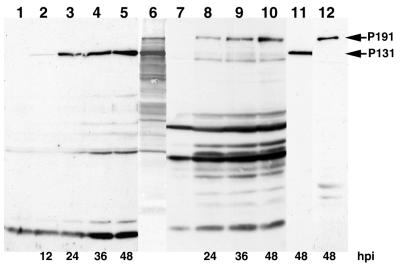
Polyclonal anti-P131 and anti-P191 sera raised against the C termini of the corresponding proteins were used to detect the two proteins in Western blots of proteins extracted from PCV-infected BY-2 protoplasts at different times postinfection. The longest polypeptide detected by the anti-P131 antibodies (Fig. 1, lanes 2 to 5) and by the anti-P191 antibodies (Fig. 1, lanes 8 to 10) each comigrated with the corresponding 35S-labeled 131K and 191K products translated in vitro from viral PCV RNA (Fig. 1, lane 6). Each of these species accumulated in increasing amounts with time after infection, and neither was detected in proteins extracted from mock-inoculated protoplasts (Fig. 1, lanes 1 and 7). In addition to the full-length P131 and P191 species, the P131- and P191-specific antisera also immunoreacted with a number of shorter polypeptides on the Western blot. However, when the antisera were further purified by affinity chromatography, P131 and P191 were still readily detected, but the immunoreactions with most of the smaller species were eliminated (Fig. 1, lanes 11 and 12). It is important to note that the anti-P131 antibodies did not detectably cross-react with P191 even though P191 contains the entire sequence of P131. This may indicate that the C terminus of P131 is not accessible in P191.
FIG. 1.
Time course of P131 and P191 synthesis in BY-2 tobacco protoplasts. Protein extracts of mock-infected (lanes 1 and 7) or PCV-infected protoplasts (lanes 2 to 5 and lanes 8 to 12) harvested at the time (hours) postinfection indicated at the bottom were analyzed by Western blotting. The proteins were separated by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis, transferred onto an Immobilon-P membrane, and probed with antisera raised against P131 (lanes 1 to 5) or P191 (lanes 7 to 10) or with affinity chromatography-purified anti-P131 (lane 11) or anti-P191 (lane 12) antibodies. Bound antibodies were visualized by chemiluminescence. Lane 6 correponds to in vitro [35S]methionine-labeled translation products obtained after incubation of viral PCV RNA in a wheat germ extract. The positions of P131 and P191 are indicated by arrows.
Colocalization of P131 and P191 with replication sites.
In a previous study, we used BrUTP-specific antibodies to detect sites of preferential incorporation of BrUTP into neosynthesized PCV RNA in virus-infected BY-2 protoplasts (15). The putative viral RNA replication sites identified in this study consisted of punctate bodies distributed throughout the cytoplasm that were particularly abundant in the perinuclear space. In such experiments, however, it is difficult to eliminate the possibility that neosynthesized BrUTP-containing RNA (Br-RNA) detaches from the replication complex and delocalizes from authentic replication sites. Therefore, we have also used an anti-dsRNA primary polyclonal antibody (28, 30) to detect the dsRNA replicative form and replicative intermediates produced during viral RNA replication (9), and we have compared the sites of dsRNA and of neosynthesized BrUTP-labeled RNA by dual-label immunofluorescence. In PCV-infected protoplasts, the distribution of Br-RNA (green spots, Fig. 2A) and of dsRNA (red spots, Fig. 2B), observed at 24 hpi, was similar, whereas no labeling was visible in mock-inoculated protoplasts (Fig. 2D and E). Superimposition of the images (Fig. 2C) detected a few green spots without corresponding red spots, but the coincidence of the majority of the Br-RNA foci with those of dsRNA confirmed that, at least for short BrUTP labeling periods, most of the Br-RNA is present at replication sites.
FIG. 2.
(Top). Localization of Br-RNA and dsRNA in typical PCV-infected BY-2 tobacco protoplasts. PCV-infected (A, B, and C) or mock-inoculated (D and E) protoplasts were treated with actinomycin D added at 17 hpi and then incubated for 1 h prior to addition of BrUTP and further incubated for 6 h. The protoplasts were then immunolabeled with mouse BrUTP-specific antibodies and dsRNA guinea pig antibodies, which were then detected with, respectively, Alexa 488 (A and D)- and Alexa 568 (B and E)-labeled secondary antibodies. BrUTP labeling is in green, and dsRNA labeling is in red. (C) Superimposition of the images to the left. The confocal images were collected with a focal depth of 0.45 μm. Bar, 10 μm.
To test whether the two presumed replication proteins, P131 and P191, are associated with active RNA synthesis sites, viral RNA-infected BY-2 protoplasts were analyzed by dual-label immunofluorescence with the purified anti-P131 (Fig. 3A and D) or anti-P191 (Fig. 3G and J) antibodies and antibodies against either Br-RNA (Fig. 3B and H) or dsRNA (Fig. 3E and K). At 24 hpi, P131 and P191 were visualized as irregularly shaped perinuclear spots (Fig. 3A and G). At a later time (48 hpi), the immunofluorescent structures increased in size and formed aggregates which, while remaining essentially perinuclear, also protruded notably into the cytoplasm (Fig. 3D and J). No signal was visible in uninfected cells processed and imaged in parallel (not shown). Staining by anti-BrUTP or by anti-dsRNA antibodies showed the same subcellular localization of viral replication sites, essentially around the nucleus. The labeling appeared in small foci at 24 hpi (Fig. 3B and H) and in large areas which seem to be composed of numerous small foci at 48 hpi (Fig. 3E and K). Superimposition of images showed extensive coincidence of the Br-RNA- and dsRNA-labeled sites with both P131 and P191 (Fig. 3C, F, I, and L). These results show that both P131 and P191 belong to the replication complex and must therefore be colocalized, although it was not possible to visualize their colocalization by dual labeling, since both the anti-P131 and anti-P191 antibodies originated from rabbits.
FIG. 3.
(Bottom). Colocalization of incorporated BrUTP or dsRNA with P131 or P191 in BY-2 protoplasts. PCV-infected protoplasts were processed for double-label immunofluorescence. P131 (A and D) and P191 (G and J) were detected by indirect immunofluorescence (in green) with purified rabbit antibodies raised against P131 or P191. The distribution of BrUTP (B and H) at 24 hpi and of dsRNA (E and K) at 48 h pi was imaged in the same 0.45-μm optical section as for P131 and P191 and is shown in red. The right column (C, F, I, and L) shows digital superimposition of the two panels to the left. Bar, 10 μm.
P131 and P191 proteins colocalize with ER-derived membranes but not with the Golgi membranes.
The perinuclear localization of the replication proteins and of dsRNA suggested a possible association with the ER. To examine more precisely the nature of the membrane compartment involved, protoplasts from transgenic ER-GFP BY-2 cells were used. In these protoplasts, transgenic GFP containing an ER targeting signal is constitutively expressed so that the ER system is visible in ER-GFP protoplasts. Typically, such protoplasts possess a three-dimensional green fluorescent network of continuous tubules and sheets that underlies the plasma membrane, courses through the cytoplasm, and links up with the nuclear envelope (7, 53). This distribution of green fluorescence was not modified when the protoplasts were fixed and processed for immunostaining (Fig. 4A). When PCV RNA-infected ER-GFP protoplasts were observed at 24 hpi, the cytoplasmic ER network largely disappeared and was replaced by fluorescent bodies which accumulated around the nucleus, whereas the nuclear envelope and the cortical ER network remained intact (Fig. 4C). These perinuclear structures, visible as early as 24 hpi, increased in size with the time of infection and invaded the nonperinuclear cytoplasm at 48 hpi (Fig. 4F). Immunostaining of protoplasts at 24 hpi with anti-P131 antibodies (Fig. 4D) or at 48 hpi with anti-P191 antibodies (Fig. 4G) showed aggregates of red fluorescent spots that accumulated around the nucleus. They increased in size and number with time postinfection, forming more diffuse packets, which mainly corresponded to ER fluorescent bodies, as is visible in the image superimposition (Fig. 4E and H). Healthy protoplasts treated similarly showed no fluorescence (Fig. 4B). Careful observation of these images reveals that both types of fluorescence are most concentrated in the center of the aggregates, but red foci corresponding to the proteins are also abundant at the perimeter of the aggregates. When the protoplasts were infected with a transcript of RNA-1 (T1) rather than viral RNA, the same ER aggregates were observed (not shown), demonstrating that the RNA-2-encoded proteins are not involved in the induced modifications of the ER and suggesting that viral RNA-1 replication per se or synthesis of one or more RNA-1 proteins is implicated in the ER modifications.
FIG. 4.
(Left). Localization of P131 and P191 in transgenic BY-2 protoplasts. Healthy (A and B) or PCV-infected (C to H) ER-GFP protoplasts expressing a GFP targeted to the ER were processed for fluorescence with primary antiserum against P131 at 24 hpi (B and D) or against P191 at 48 hpi (G). Similarly, healthy (I and J) or infected (K to P) Man1::GFP protoplasts expressing a GFP targeted to the Golgi apparatus were processed to visualize the P131 (J and L) or the P191 (O) accumulation sites. The digital superimposition of both fluorescent signals is shown in panels E, H, M, and P. Bar, 10 μm.
To further examine the nature of the membranes associated with the viral RNA replication complex, similar experiments were performed with protoplasts prepared from Man1::GFP transgenic BY-2 tobacco cells. These protoplasts constitutively express GFP fused to α-1,2 mannosidase, a protein confined to the cis side of the Golgi stacks (36). As shown (Fig. 4I), such protoplasts contain numerous green fluorescent points corresponding to individual Golgi stacks scattered throughout the cytoplasm and at the cell perimeter. In infected protoplasts (Fig. 4K and N), the number and distribution of the Golgi stacks did not differ from those of noninfected protoplasts. In the infected Man1::GFP protoplasts, the pattern of red immunofluorescence staining obtained with anti-P131 (Fig. 4L) or anti-P191 antibodies (Fig. 4O) rarely, if ever, coincided with Golgi stacks, as shown by the superimposition of images (Fig. 4M and P). Observation of the protoplasts with an increased gain of the confocal microscope photoreceptor often revealed, in addition to the fluorescent Golgi bodies, faint perinuclear GFP fluorescence (Fig. 4N), which coincides with the perinuclear P191 localization (Fig. 4O) and which therefore probably corresponds to the P191/ER aggregates. This fluorescence may reflect decreased transport of the α-1,2 mannosidase-GFP from the ER to the Golgi apparatus, consequent to the ER perturbations induced by virus infection.
P15 is not colocalized with P131 and the ER.
We showed previously that EGFP/P15 is not detected at viral RNA replication sites (15). To further explore the localization of P15 relative to the replication complex, we compared its distribution relative to P131 and to the ER. First, the transcript TRep-EG15, expressing an EGFP/P15 fusion protein, was inoculated to wild-type protoplasts together with the transcripts T1 and T2, corresponding to RNA-1 and -2 (15). At 24 hpi the protoplasts incubated with BrUTP were processed for immunostaining with anti-BrUTP (red, Fig. 5B) and anti-P131 (white, Fig. 5C) antisera. As predicted, the EGFP fluorescence corresponding to P15 (green, Fig. 5A) was associated neither with Br-RNA (Fig. 5D) nor with P131 (Fig. 5E), whereas Br-RNA and P131 colocalized quite well (Fig. 5F).
FIG. 5.
(Right). Localization of P15 compared with that of Br-RNA, P131, and ER. The first row of images shows the same ER-GFP transgenic protoplast infected with T1, T2, and TRep-EG15 processed at 24 hpi. Green fluorescence of EGFP/P15 fusion protein is shown in panel A. Primary antibodies against Br-RNA and P131 were revealed, respectively, by Alexa 568 (red, B) and Alexa 633 (white, C) secondary antibody. Digital superimposition of images from the same 0.45-μm optical section shows Br-RNA and EGFP/P15 (D), P131 and EGFP/P15 (E), and P131 and Br-RNA (F). The third and fourth rows correspond to the same representative ER-GFP transgenic protoplast infected with T1, T2, and TRep-RFP15 (red) in two different optical sections at 48 hpi. Green ER-GFP fluorescence (G and J) and red RFP/P15 fluorescence (H and K) are superimposed in panels I and L. Bar, 10 μm.
To visualize the intracellular distribution of P15, ER-GFP protoplasts infected with T1, T2, and TRep-RFP15, which expresses a Discosoma red fluorescent protein fused to P15 (RFP/P15), were observed at 48 hpi. P15 (red) rarely colocalized with the ER aggregates (green) but was present in the same areas and often appeared at the periphery of the condensate ER, as observed on the two optical sections of the same protoplast shown in Fig. 5 G to L. When Man1::GFP transgenic protoplasts were similarly infected, the fluorescent punctate bodies of RFP/P15 did not coincide with the Golgi GFP labeling (not shown).
Effect of cerulenin and brefeldin A (BFA) on PCV replication.
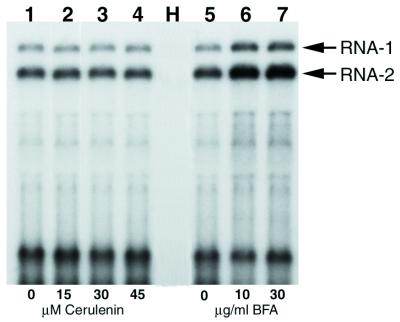
In the case of poliovirus and cowpea mosaic virus (CPMV), cerulenin, a fungal antibiotic which prevents de novo phospholipid synthesis, was shown to inhibit both viral RNA replication and proliferation of ER, suggesting that de novo membrane synthesis is needed to promote viral RNA replication (11, 19, 38). To test the effect of cerulenin on PCV replication, PCV-infected protoplasts were divided into four aliquots, and the drug was added to the incubation medium at three different concentrations. Northern blot analysis of RNA extracted at 48 hpi showed that the yield of viral RNAs was similar in samples incubated with 15, 30, or 45 μM cerulenin (Fig. 6, lanes 2 to 4) as in the sample incubated without cerulenin (Fig. 6, lane 1). The same result was obtained when the analysis was performed at 12 and 24 hpi (not shown). Since multiplication of grapevine fanleaf virus was found to be affected by a similar cerulenin treatment of BY-2 protoplasts (unpublished data), we conclude that cerulenin penetrates into protoplasts but has no effect on PCV replication. These observations suggest that the ER aggregates observed in PCV-infected protoplasts correspond to a reorganization of preexisting membranes induced by RNA replication rather than to proliferation of de novo-synthesized membranes. Similar findings have been reported for AMV and Tobacco mosaic virus (TMV) (11).
FIG. 6.
Analysis of the accumulation of viral RNA in drug-treated PCV-infected protoplasts. BY-2 tobacco protoplasts inoculated with PCV RNA were either untreated (lanes 1 and 5) or treated with 15, 30, or 45 μM cerulenin (lanes 2, 3, and 4) or with 10 or 30 μg of BFA/ml (lanes 6 and 7). The drug was added just after infection, and the protoplasts were harvested at 48 hpi. H corresponds to total RNA extracted from uninfected protoplasts. Blots of extracted total RNA were probed with a 32P-labeled riboprobe corresponding to the complementary sequence of 3′-terminal 124 nucleotides common to both PCV genomic RNAs. The positions of RNA-1 and RNA-2 are indicated by arrows.
We also investigated the effect on PCV replication of BFA, a fungal metabolite which is currently used to study membrane traffic in eukaryotic cells. BFA inhibits the processing and transport of glycoproteins of enveloped viruses (12, 13, 54, 59), but it has also been shown to inhibit the genome replication of poliovirus, suggesting that the assembly and function of the replication complex require vesicular transport through the Golgi complex (26, 34). BY-2 protoplasts infected with viral RNA were incubated in the absence of BFA or with 10 or 30 μg of BFA/ml added immediatly after infection or 6 h later. The accumulation of viral RNA was analyzed by Northern blotting at 24 or 48 hpi. Figure 6 (lanes 6 and 7) shows the results obtained when BFA was added immediately after infection of the protoplasts and when the RNA was extracted at 48 hpi. The amount of viral RNA detected in the presence of BFA was generally two times higher than in the absence of BFA (Fig. 6, lane 5). This suggests that disruption of the Golgi apparatus and its fusion to the ER induced by BFA (22, 46, 49) not only does not inhibit but in fact stimulates PCV RNA accumulation. Similar results were obtained under the other BFA treatment conditions mentioned above (data not shown).
ER modification in infected plants.
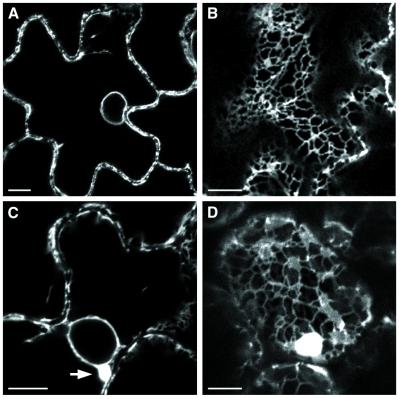
To investigate whether the ER modifications observed in protoplasts also occur in cells from infected host plants, apical leaves of transgenic N. benthamiana plants expressing GFP targeted to the lumen of the ER were examined by confocal microscopy at 12 days postinfection. In the epidermal cells of mock-inoculated plants (Fig. 7A and B), the typical ER network, the nuclear envelope (Fig. 7A), and the ER tubules traversing the cytoplasmic threads (Fig. 7B) were green fluorescent. In epidermal cells of systemically infected leaves (Fig. 7C and D), perinuclear green fluorescent bodies (Fig. 7C, arrow) were visible in many cells, showing that the infection resulted in the formation of ER membrane aggregates such as those observed in infected protoplasts. Moreover, in most cases, the cortical ER network remained visible (Fig. 7D) and appeared not to have been modified.
FIG. 7.
Confocal fluorescence micrograph of mock-infected (A and B) or PCV-infected (C and D) epidermal cells of ER-GFP transgenic N. benthamiana. (A) Perinuclear and plasma membranes show a fluorescent halo in a healthy ER-GFP N. benthamiana. (B) Normal reticulate pattern of cortical ER in apical leaf cell of mock-inoculated plants. (C) Perinuclear ER aggregates (arrow) observed in epidermal cells of PCV-infected systemic leaves. (D) Cortical fluorescent bodies and typical ER network in infected leaves. Bar, 10 μm.
Ultrastructural analysis of PCV-infected BY-2 protoplasts.
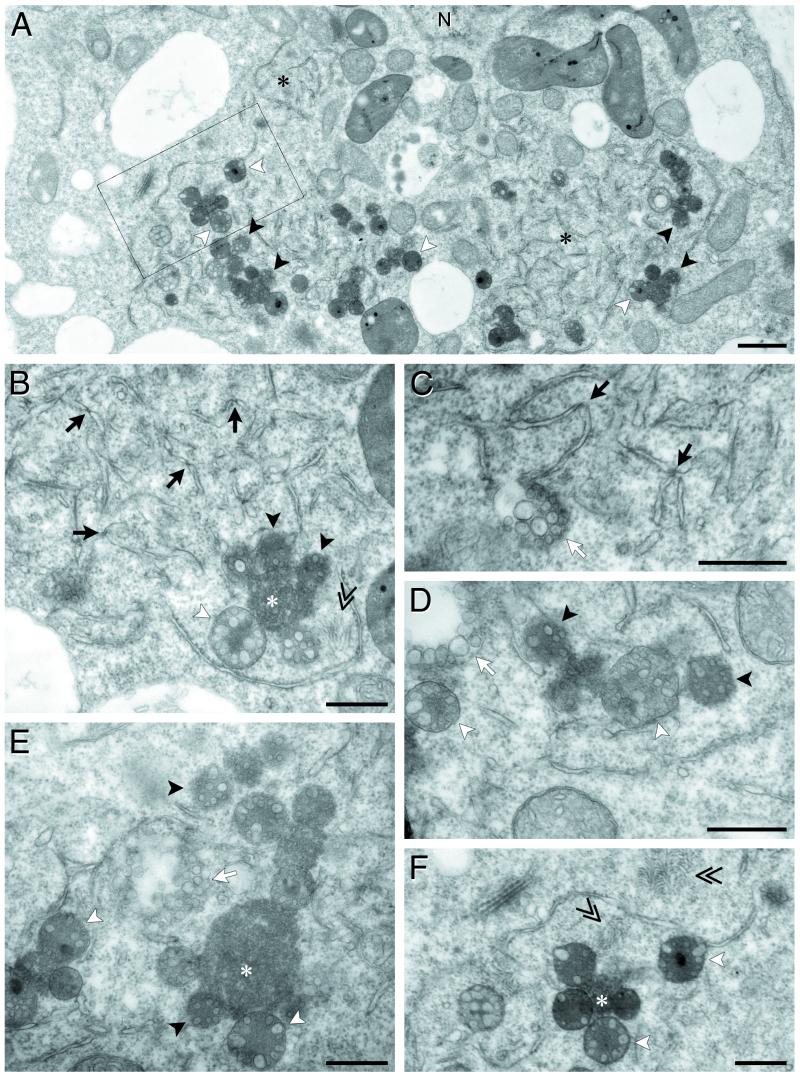
To obtain further information concerning the PCV-induced cytopathic effects visualized by fluorescence microscopy, ultrathin sections of chemically fixed resin-embedded protoplasts harvested at 48 hpi were observed by electron microscopy (EM). At low magnification, infected protoplasts were easy to identify due to the presence of defined regions in which gross ultrastructural alterations of cellular components were detected (Fig. 8). These areas were generally located in the vicinity of the nucleus and clearly contained not only globose bodies (Fig. 8A, black and white arrowheads) but also modified ER (Fig. 8A, black asterisks), as suspected from the laser-scanning confocal miscroscopy studies. Identification of the ER membranes was based on the presence of ribosomes clearly visible on contiguous membrane regions shown at the higher magnification in Fig. 8B. In this figure, alteration of the ER is also readily apparent; instead of forming a well-organized network of bilayered membranes lamellae and tubules, ER cisternae appeared to be fragmented into pieces (ranging from 0.5 to 1.2 μm in length) due to severe constrictions at defined points that appear to be more electron dense (Fig. 8B and C, black arrows). Vesicles of 80 to 200 nm were frequently associated with the regions containing “pinched” ER fragments and occasionally accumulated into small clusters near the ER fragments (Fig. 8C, D, and E, white arrows). These observations suggest that these vesicles may derive from the ER. The other consistent feature of PCV-infected BY-2 protoplasts was the presence in the cytoplasm of globose multivesicular bodies (MVB) (Fig. 8A, B, D, E, and F, white and black arrowheads). These MVB varied in opacity ranging from very electron-dense structures to much lighter structures (Fig. 8A, B, and F) and also varied in size but were clearly distinct from mitochondria or plastids. Two main types of morphology were observed for the MVB. Some contained a dense matrix and a disordered accumulation of membranous vesicles (Fig. 8A, B, D, and E, black arrowheads) while others contained a central core of granular material surrounded by, at least, one layer of membranous vesicles (Fig. 8A, B, D, E, and F, white arrowheads). The outer envelope of these MVB was not always well defined. Whereas some MVB displayed a rather diffuse envelope (black arrowheads), others were clearly surrounded by a single membrane (white arrowheads). MVB were often found associated with other dense bodies that were generally more variable in size and in which no vesicles were detectable (Fig. 8B, E, and F, white asterisks). Finally, another consistent feature of the PCV-infected BY-2 protoplasts was the presence of clusters of virions (Fig. 8B and F, double arrowheads). None of these cytopathic structures were ever observed in sections from healthy BY-2 protoplasts (data not shown).
FIG. 8.
Electron microscopy of cytopathological structures in PCV-infected BY-2 protoplasts at 48 hpi. (A) Low-magnification view of the ultrastructural modifications. (B, C, D, E, and F) Higher-magnification views of the different cytopathic structures. Black asterisks correspond to area of broken ER, black arrows point to constrictions on the ER fragments, and white arrows indicate clusters of vesicles. Single arrowheads correspond to MVB; MVB containing disordered membranous vesicles are indicated by black arrowheads, whereas those containing one row of vesicles and surrounded by a single membrane are indicated by white arrowheads. White asterisks correspond to electron-dense material without detectable vesicles. Double arrows indicate clusters of virions. (F) Magnification of the inset square indicated in panel A. N, nucleus. Bars, 1 μm in panel A and 0.5 μm in panels B, C, D, E, and F.
DISCUSSION
In this study, dual-labeled immunofluorescence was used to demonstrate that, in PCV-infected protoplasts, both P131 and P191 colocalize with dsRNA and neosynthesized RNA and thus are present at active replication sites, in full agreement with their assumed functions in PCV replication. This suggests that, like the brome mosaic virus 1a and 2a proteins (44, 45) and the AMV P1 and P2 proteins (55), the two PCV replication proteins colocalize and are components of the replication complex. On the contrary, P15 and the replication proteins never colocalized, confirming that P15 does not belong to the replication complex (15). Fluorescence microscopy of ER-GFP transgenic BY-2 protoplasts revealed extensive reorganization of the ER upon infection by PCV RNA compared to healthy protoplasts. These modifications concerned essentially the ER surrounding the nucleus and start with the formation of small bodies observed at early stages of infection. The replication proteins always localize with the ER bodies, and their parallel increase in number and size demonstrates that the modifications of the ER are correlated with the replication process. The same cytopathic modifications were produced in protoplasts infected with RNA-1 transcript alone, indicating that they were induced by one or more of the RNA-1-encoded proteins or directly by ongoing replication. Recruitment of the ER membranes for RNA replication has been reported for many plant viruses (8, 11, 32, 45, 50), although the perinuclear aggregates generally observed may originate from different compartments of the ER or from de novo membrane synthesis. In the case of PCV, the formation of large perinuclear ER aggregates in infected protoplasts and in infected N. benthamiana epidermal cells does not occur at the expense of the cortical compartment of the ER, in contrast to what was observed for TMV (42). On the other hand, treatment with cerulenin had no effect on PCV replication in protoplasts, suggesting that, in contrast to CPMV, there is no requirement for de novo lipid biosynthesis (11). Furthermore, BFA did not inhibit PCV replication. Treatment of BY-2 tobacco cells with BFA results in the inhibition of the retrograde transport by disruption of COPI vesicles which deliver their cargo to the ER and produces a complete disruption of the secretory system (46). Therefore, COPI vesicles or functional exocytosis pathways are very unlikely to play a role in PCV replication. This is consistent with the fact that the distribution of the Golgi stacks was not significantly modified by PCV, at least at an early stage of infection. Indeed, our results showed that BFA treatment slightly enhanced PCV replication. The fact that, at a late time of infection, we visualized a faint Golgi-specific fluorescence which colocalized with ER aggregates suggests that PCV infection leads to a partial redistribution of the Golgi apparatus in the ER. Thus, BFA treatment may increase viral RNA synthesis because it contributes to membrane modifications induced by PCV or modifies the content of ER cellular factors involved in PCV replication. All of these results thus suggest that recruitment of membranes by PCV replication differs from TMV and CPMV.
From our electron microscopic observations, it appears that the ER near the nucleus is fragmented and vesiculated. This fragmentation probably safeguards the cortical ER and accounts for the condensed appearance of the perinuclear ER in fluorescence observations. A swelling of the ER at the ends of the broken fragments was also frequently observed in the electron micrographs. These may be budding sites and could be at the origin of some of the vesicles and aggregates of vesicles which were observed, although we cannot rule out the possibility that they also arise from modifications of other organelles present in the cytoplasm.
We have shown by fluorescence microscopy that P131 or P191 mainly colocalizes in the central region of the green fluorescent bodies formed by the ER, although some spots corresponding to P131 or P191 were also detected at the periphery of these bodies, particularly at 48 hpi. On the other hand, electron microscopic images showed globose bodies containing numerous vesicles at the periphery of the areas of broken ER. As noted in the introduction, viral RNA replication sites frequently colocalize with vesicles that have different membranous origins (1, 4, 14, 17, 28, 29, 37, 48). The MVB associated with tombusvirus infections consist of a main body surrounded by ovoid vesicles measuring 80 to 150 nm in diameter. The presence of dsRNA within these vesicles and their association with proteins of the replicase complex provide evidence that the tombusvirus MVB are the sites of virus replication (1, 14, 48). Among the different cytopathic structures detected in PCV-infected BY-2 protoplasts, the MVB surrounded by a single membrane strongly resemble the MVB induced by cymbidium ringspot virus and tomato bushy stunt virus infections, which have been shown to be modified peroxisomes (10, 16, 47, 48). P15, which is essential for efficient viral RNA accumulation, has recently been shown to act as a suppressor of posttranscriptional gene silencing (P. Dunoyer et al., unpublished data). P15 contains at its C terminus the triplet SKL, which corresponds to the canonical type 1 peroxisomal targeting signal (PTS1). By fluorescence microscopy, GFP-labeled P15 has been detected in punctate structures which are sometimes localized close to the ER aggregates. We have recently demonstrated that these punctate structures correspond to peroxisomes (Dunoyer et al., unpublished). We hypothesize that some of the PCV MVB described here are modified peroxisomes and contain P15. If so, the replication complexes are presumably present in one of the other cytopathic structures.
Our findings suggest that, like the flaviviruses (29, 58), ER modifications and MVB induced by PCV infection correspond to a complex replication factory in which defined areas are assigned special functions. Additional experiments will evidently be necessary to better define the nature of the ER modifications and of the MVB induced by PCV infection, to localize the replication complex more precisely, and to determine the role of the posttranscriptional gene silencing suppressor P15 in the process.
Acknowledgments
We thank K. Danna and A. Nebenführ for providing the transgenic BY-2 cell lines, D. Baulcombe for ER-GFP transgenic N. benthamiana, and J. Y. Lee for anti-dsRNA antibodies. We also thank O. Rohfritsch for help with the electron microscopy and P. Pfeiffer and C. Garaud for helpful suggestions and advice at various stages. We are also grateful to K. Richards for critically reading the manuscript.
The Inter-Institut confocal microscopy platform was cofinanced by the CNRS, the Université Louis Pasteur, the Région Alsace, the Association pour la Recherche sur le Cancer (ARC), and the Ligue de la Recherche sur le Cancer.
REFERENCES
- 1.Appiano, A., G. D’Agostino, M. Bassi, N. Barbieri, G. Viale, and P. Dell’Orto. 1986. Origin and function of tomato bushy stunt virus-induced inclusion bodies. II. Quantitative electron microscope autoradiography and immunogold cytochemistry. J. Ultrastruct. Res. 97:31–38. [Google Scholar]
- 2.Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220–226. [DOI] [PubMed] [Google Scholar]
- 3.Bienz, K., D. Egger, and T. Pfister. 1994. Characteristics of the poliovirus replication complex. Arch. Virol. Suppl. 9:147–157. [DOI] [PubMed] [Google Scholar]
- 4.Bienz, K., D. Egger, T. Pfister, and M. Troxler. 1992. Structural and functional characterization of the poliovirus replication complex. J. Virol. 66:2740–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz, K., D. Egger, Y. Rasser, and W. Bossart. 1983. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology 131:39–48. [DOI] [PubMed] [Google Scholar]
- 6.Bienz, K., D. Egger, M. Troxler, and L. Pasamontes. 1990. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J. Virol. 64:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boevink, P., K. Oparka, S. Santa Cruz, B. Martin, A. Betteridge, and C. Hawes. 1998. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 15:441–447. [DOI] [PubMed] [Google Scholar]
- 8.Boevink, P., S. Santacruz, C. Hawes, N. Harris, and K. J. Oparka. 1996. Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J. 10:935–941. [Google Scholar]
- 9.Buck, K. W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgyan, J., L. Rubino, and M. Russo. 1996. The 5′-terminal region of a tombusvirus genome determines the origin of multivesicular bodies. J. Gen. Virol. 77:1967–1974. [DOI] [PubMed] [Google Scholar]
- 11.Carette, J. E., M. Stuiver, J. van Lent, J. Wellink, and A. B. van Kammen. 2000. Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis. J. Virol. 74:6556–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, S. Y., Y. Matsuoka, and R. W. Compans. 1991. Assembly and polarized release of Punta Toro virus and effects of brefeldin A. J. Virol. 65:1427–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung, P., B. W. Banfield, and F. Tufaro. 1991. Brefeldin A arrests the maturation and egress of herpes simplex virus particles during infection. J. Virol. 65:1893–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Franco, A., M. Russo, and G. P. Martelli. 1984. Ultrastructure and origin of cytoplasmic multivesicular bodies induced by carnation Italian ringspot virus. J. Gen. Virol. 65:1233–1237. [Google Scholar]
- 15.Dunoyer, P., E. Herzog, O. Hemmer, C. Ritzenthaler, and C. Fritsch. 2001. Peanut clump virus RNA-1-encoded P15 regulates viral RNA accumulation but is not abundant at viral RNA replication sites. J. Virol. 75:1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francki, R. I. B., R. G. Milne, and T. Hatta. 1985. Tombusvirus group, p.181–197. Atlas of plant viruses, vol. 1. CRC Press, Inc., Boca Raton, Fla.
- 17.Froshauer, S., J. Kartenbeck, and A. Helenius. 1988. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J. Cell Biol. 107:2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnier, M., T. Candresse, and J. M. Bové. 1986. Immunocytochemical localization of TYMV-coded structural and nonstructural proteins by the protein A-gold technique. Virology 151:100–109. [DOI] [PubMed] [Google Scholar]
- 19.Guinea, R., and L. Carrasco. 1990. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 9:2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haseloff, J., P. Goelet, D. Zimmern, P. Ahlquist, R. Dasgupta, and P. Kaesberg. 1984. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc. Natl. Acad. Sci. USA 81:4358–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatta, T., and R. I. B. Francki. 1981. Cytopathic structures associated with tonoplasts of plant cells infected with cucumber mosaic and tomato aspermy viruses. J. Gen. Virol. 53:343–346. [Google Scholar]
- 22.Hawes, C. R., F. Brandizzi, and A. V. Andreeva. 1999. Endomembranes and vesicle trafficking. Curr. Opin. Plant Biol. 2:454–461. [DOI] [PubMed] [Google Scholar]
- 23.Hemmer, O., C. Oncino, and C. Fritsch. 1993. Efficient replication of the in vitro transcripts from cloned cDNA of tomato black ring virus satellite RNA requires the 48K satellite RNA-encoded protein. Virology 194:800–806. [DOI] [PubMed] [Google Scholar]
- 24.Herzog, E., H. Guilley, S. K. Manohar, M. Dollet, K. Richards, C. Fritsch, and G. Jonard. 1994. Complete nucleotide sequence of peanut clump virus RNA 1 and relationships with other fungus-transmitted rod-shaped viruses. J. Gen. Virol. 75:3147–3155. [DOI] [PubMed] [Google Scholar]
- 25.Herzog, E., O. Hemmer, S. Hauser, G. Meyer, S. Bouzoubaa, and C. Fritsch. 1998. Identification of genes involved in replication and movement of peanut clump virus. Virology 248:312–322. [DOI] [PubMed] [Google Scholar]
- 26.Irurzun, A., L. Perez, and L. Carrasco. 1992. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology 191:166–175. [DOI] [PubMed] [Google Scholar]
- 27.Koonin, E. V., V. P. Boyko, and V. V. Dolja. 1991. Small cysteine-rich proteins of different groups of plant RNA viruses are related to different families of nucleic acid-binding proteins. Virology 81:395–398. [DOI] [PubMed] [Google Scholar]
- 28.Lee, J. Y., J. A. Marshall, and D. S. Bowden. 1994. Characterization of rubella virus replication complexes using antibodies to double-stranded RNA. Virology 200:307–312. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie, J. M., M. K. Jones, and E. G. Westaway. 1999. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J. Virol. 73:9555–9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magliano, D., J. A. Marshall, D. S. Bowden, N. Vardaxis, J. Meanger, and J. Y. Lee. 1998. Rubella virus replication complexes are virus-modified lysosomes. Virology 40:57–63. [DOI] [PubMed] [Google Scholar]
- 31.Manohar, S. K., H. Guilley, M. Dollet, K. Richards, and G. Jonard. 1993. Nucleotide sequence and genetic organization of peanut clump virus RNA 2 and partial characterization of deleted forms. Virology 195:33–41. [DOI] [PubMed] [Google Scholar]
- 32.Mas, P., and R. N. Beachy. 1999. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J. Cell Biol. 147:945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda, D., P. Dunoyer, O. Hemmer, C. Fritsch, and T. W. Dreher. 2000. The valine anticodon and valylatability of Peanut clump virus RNAs are not essential but provide a modest competitive advantage in plants. J. Virol. 74:8720–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maynell, L. A., K. Kirkegaard, and M. W. Klymkowsky. 1992. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 66:1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagata, T., Y. Nemoto, and S. Hasezawa. 1992. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int. Rev. Cytol. 132:1–30. [Google Scholar]
- 36.Nebenführ, A., L. A. Gallagher, T. G. Dunahay, J. A. Frohlick, A. M. Mazurkiewicz, J. B. Meehl, and L. A. Staehelin. 1999. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 121:1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng, M. L., J. S. Pedersen, B. H. Toh, and E. G. Westaway. 1983. Immunofluorescent sites in vero cells infected with the flavivirus Kunjin. Arch. Virol. 78:177–190. [DOI] [PubMed] [Google Scholar]
- 38.Omura, S. 1976. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol. Rev. 40:681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peränen, J., and L. Kääriäinen. 1991. Biogenesis of type I cytopathic vacuoles in semliki forest virus-infected BHK cells. J. Virol. 65:1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peränen, J., P. Laakkonen, M. Hyvönen, and L. Kääriäinen. 1995. The alphavirus replicase protein nsP1 is membrane-associated and has affinity to endocytic organelles. Virology 208:610–620. [DOI] [PubMed] [Google Scholar]
- 41.Prod’homme, D., S. Le Panse, G. Drugeon, and I. Jupin. 2001. Detection and subcellular localization of the turnip yellow mosaic virus 66K replication protein in infected cells. Virology 281:88–101. [DOI] [PubMed] [Google Scholar]
- 42.Reichel, C., and R. N. Beachy. 1998. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 95:11169–11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Restrepo, M. A., D. D. Freed, and J. C. Carrington. 1990. Nuclear transport of plant potyviral proteins. Plant Cell 2:987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Restrepo-Hartwig, M., and P. Ahlquist. 1999. Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum. J. Virol. 73:10303–10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Restrepo-Hartwig, M. A., and P. Ahlquist. 1996. Brome mosaic virus helicase- and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J. Virol. 70:8908–8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritzenthaler, C., A. Nebenführ, A. Movafeghi, C. Stussi-Garaud, L. Behnia, P. Pimpl, L. A. Staehelin, and D. G. Robinson. Reevaluation of the effects of brefeldin A on plant cells using tobacco BY-2 cells expressing Golgi-targeted GFP and COPI-antisera. Plant Cell, in press. [DOI] [PMC free article] [PubMed]
- 47.Rubino, L., and M. Russo. 1998. Membrane targeting sequences in tombusvirus infections. Virology 252:431–437. [DOI] [PubMed] [Google Scholar]
- 48.Russo, M., A. Di Franco, and G. P. Martelli. 1983. The fine structure of Cymbidium ringspot virus infections in host tissues. III. Role of peroxisomes in the genesis of multivesicular bodies. J. Ultrastruct. Res. 82:52–63. [DOI] [PubMed] [Google Scholar]
- 49.Satiat-Jeunemaitre, B., L. Cole, T. Bourett, R. Howard, and C. Hawes. 1996. Brefeldin A effects in plant and fungal cells: something new about vesicle trafficking? J. Microsc. 181:162–177. [DOI] [PubMed] [Google Scholar]
- 50.Schaad, M. C., P. E. Jensen, and J. C. Carrington. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlegel, A., T. H. Giddings, M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholthof, K. B. G., H. B. Scholthof, and A. O. Jackson. 1995. The tomato bushy stunt virus replicase proteins are coordinately expressed and membrane associated. Virology 208:365–369. [DOI] [PubMed] [Google Scholar]
- 53.Staehelin, L. A. 1997. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J. 11:1151–1165. [DOI] [PubMed] [Google Scholar]
- 54.Ulmer, J. B., and G. Palade. 1991. Effects of brefeldin A on the processing of viral envelope glycoproteins in murine erythroleukemia cells. J. Biol. Chem. 266:9173–9179. [PubMed] [Google Scholar]
- 55.van der Heijden, M. W., J. E. Carette, P. J. Reinhoud, A. Haegi, and J. F. Bol. 2001. Alfalfa mosaic virus replicase proteins P1 and P2 interact and colocalize at the vacuolar membrane. J. Virol. 75:1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Meer, Y., E. J. Snijder, J. C. Dobbe, S. Schleich, M. R. Denison, W. J. Spaan, and J. K. Locker. 1999. Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J. Virol. 73:7641–7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verchot, J., and J. C. Carrington. 1995. Evidence that the potyvirus P1 proteinase functions in trans as an accessory factor for genome amplification. J. Virol. 69:3668–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westaway, E. G., J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71:6650–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]