Abstract
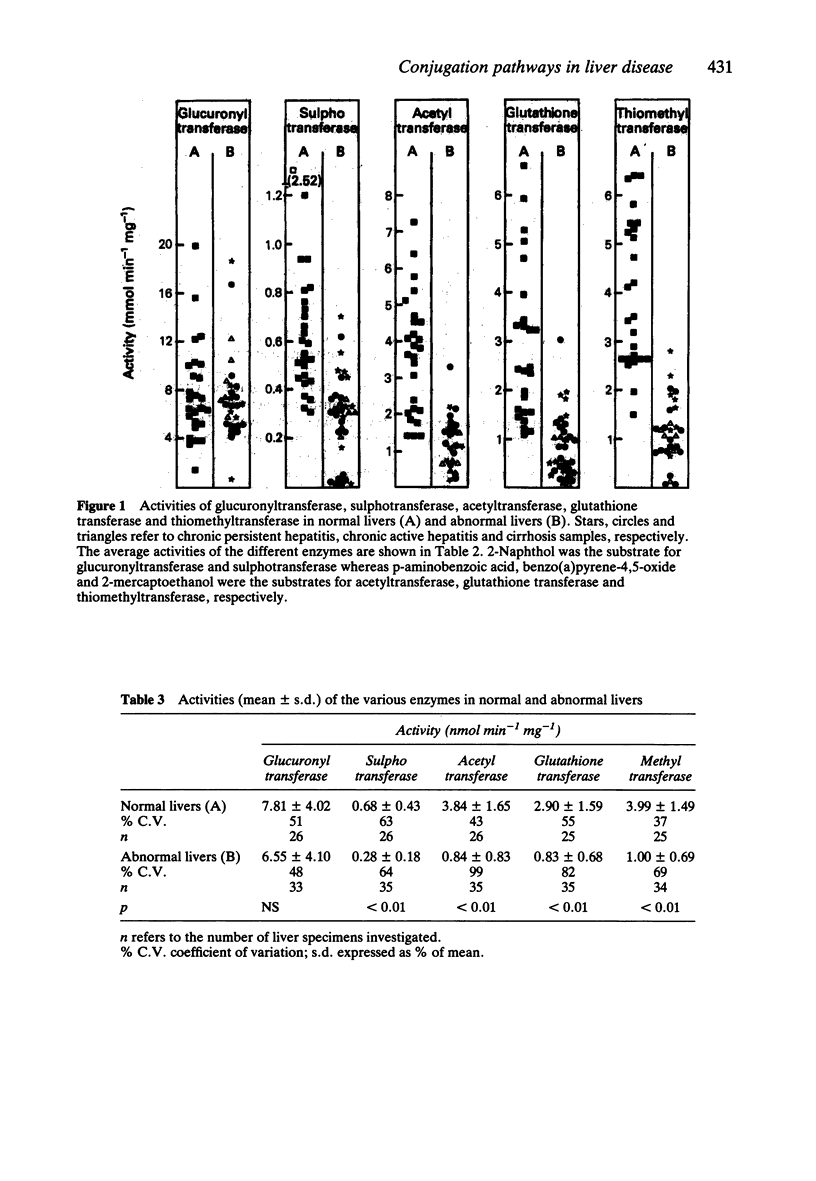
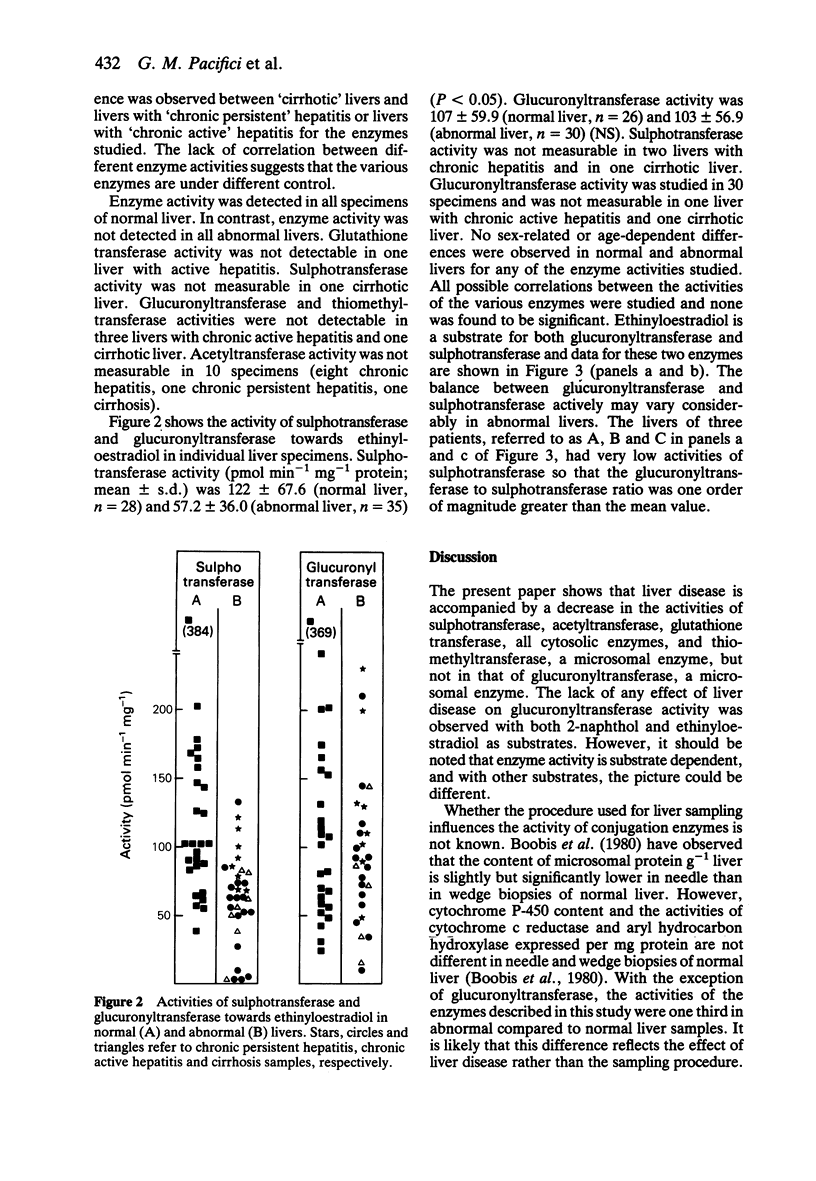
1. The activities of microsomal glucuronyltransferase and thiomethyltransferase, and those of cytosolic sulphotransferase, acetyltransferase, glutathione transferase and thiomethyltransferase were measured in abnormal (cirrhosis and chronic hepatitis) and normal livers. 2. Glucuronyltransferase and sulphotransferase were investigated with 2-naphthol and ethinyloestradiol as substrates. p-Aminobenzoic acid, benzo(a)pyrene-4,5-epoxide and 2-mercaptoethanol were the substrates of acetyltransferase, glutathione transferase and thiomethyltransferase, respectively. 3. Enzyme activities are expressed as nmol min-1 incubation mg-1 protein and the averages (+/- s.d.) are given. With 2-naphthol as substrate, the glucuronyltransferase activity was 6.55 +/- 4.10 (abnormal liver, n = 33) and 7.81 +/- 4.02 (normal liver, n = 26) (NS); whereas sulphotransferase activity was 0.28 +/- 0.18 (abnormal liver, n = 35) and 0.68 +/- 0.43 (normal liver, n = 26) (P less than 0.01). Glucuronyltransferase activity towards ethinyloestradiol was 102.5 +/- 56.9 (abnormal liver, n = 30) and 107 +/- 59.9 (normal liver, n = 26) (NS), whereas sulphotransferase activity was 57.2 +/- 36.0 (abnormal liver, n = 35) and 122 +/- 67.6 (normal liver, n = 28) (P less than 0.01). Acetyltransferase activity was 0.84 +/- 0.83 (abnormal liver, n = 35) and 3.84 +/- 1.65 (normal liver, n = 26) (P less than 0.01). Glutathione transferase activity was 0.83 +/- 0.68 (abnormal liver, n = 35) and 2.90 +/- 1.59 (normal liver, n = 25) (P less than 0.01) and thiomethyltransferase activity was 1.00 +/- 0.69 (abnormal liver, n = 34) and 3.99 +/- 1.49 (normal liver, n = 25) (P less than 0.01). 4. Liver disease lowers the activities towards the substrates studied of sulphotransferase, acetyltransferase, glutathionetransferase and thiomethyltransferase but not that of glucuronyltransferase.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Williams R. L. Guide to drug dosage in hepatic disease. Clin Pharmacokinet. 1988 Dec;15(6):396–420. doi: 10.2165/00003088-198815060-00004. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Brunner G., Hoensch H., Huber E., Josting D. Determination of microsomal UDP-glucuronyltransferase in needle-biopsy specimens of human liver. Eur J Clin Pharmacol. 1978 Dec 18;14(5):367–373. doi: 10.1007/BF00611908. [DOI] [PubMed] [Google Scholar]
- Boobis A. R., Brodie M. J., Kahn G. C., Fletcher D. R., Saunders J. H., Davies D. S. Monooxygenase activity of human liver in microsomal fractions of needle biopsy specimens. Br J Clin Pharmacol. 1980 Jan;9(1):11–19. doi: 10.1111/j.1365-2125.1980.tb04790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M. J., Boobis A. R., Bulpitt C. J., Davies D. S. Influence of liver disease and environmental factors on hepatic monooxygenase activity in vitro. Eur J Clin Pharmacol. 1981;20(1):39–46. doi: 10.1007/BF00554665. [DOI] [PubMed] [Google Scholar]
- Farrell G. C., Cooksley W. G., Powell L. W. Drug metabolism in liver disease: activity of hepatic microsomal metabolizing enzymes. Clin Pharmacol Ther. 1979 Oct;26(4):483–492. doi: 10.1002/cpt1979264483. [DOI] [PubMed] [Google Scholar]
- Howden C. W., Birnie G. G., Brodie M. J. Drug metabolism in liver disease. Pharmacol Ther. 1989;40(3):439–474. doi: 10.1016/0163-7258(89)90088-0. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Krasner N., Portmann B., Eddleston A. L., Williams R. Hepatocellular carcinoma in Great Britain: influence of age, sex, HBsAg status, and aetiology of underlying cirrhosis. Gut. 1978 Nov;19(11):1022–1026. doi: 10.1136/gut.19.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orme M. L., Back D. J., Breckenridge A. M. Clinical pharmacokinetics of oral contraceptive steroids. Clin Pharmacokinet. 1983 Mar-Apr;8(2):95–136. doi: 10.2165/00003088-198308020-00001. [DOI] [PubMed] [Google Scholar]
- Orme M. L. The third S.K. & F. Prize lecture, University of London, December 1981. The clinical pharmacology of oral contraceptive steroids. Br J Clin Pharmacol. 1982 Jul;14(1):31–42. doi: 10.1111/j.1365-2125.1982.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterness D. M., Keith R. A., Kerremans A. L., Weinshilboum R. M. Mouse liver thiol methyltransferase. Assay conditions, biochemical properties, and strain variation. Drug Metab Dispos. 1986 Nov-Dec;14(6):680–688. [PubMed] [Google Scholar]
- Pacifici G. M., Back D. J. Sulphation and glucuronidation of ethinyloestradiol in human liver in vitro. J Steroid Biochem. 1988 Sep;31(3):345–349. doi: 10.1016/0022-4731(88)90360-3. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Bencini C., Rane A. Acetyltransferase in humans: development and tissue distribution. Pharmacology. 1986;32(5):283–291. doi: 10.1159/000138181. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Franchi M., Colizzi C., Giuliani L., Rane A. Glutathione S-transferase in humans: development and tissue distribution. Arch Toxicol. 1988;61(4):265–269. doi: 10.1007/BF00364848. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Franchi M., Colizzi C., Giuliani L., Rane A. Sulfotransferase in humans: development and tissue distribution. Pharmacology. 1988;36(6):411–419. doi: 10.1159/000138330. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Temellini A., Giuliani L., Rane A., Thomas H., Oesch F. Cytosolic epoxide hydrolase in humans: development and tissue distribution. Arch Toxicol. 1988;62(4):254–257. doi: 10.1007/BF00332483. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Mamelok R. D. Hepatic disease and drug pharmacokinetics. Clin Pharmacokinet. 1980 Nov-Dec;5(6):528–547. doi: 10.2165/00003088-198005060-00002. [DOI] [PubMed] [Google Scholar]
- Woodhouse K. W., Mutch E., Williams F. M., James O. F., Rawlins M. D. 7-Ethoxyresorufin deethylase (EROD) in human liver--the effect of alcoholic liver disease. Eur J Clin Pharmacol. 1987;32(6):559–562. doi: 10.1007/BF02455988. [DOI] [PubMed] [Google Scholar]
- Woodhouse K. W., Williams F. M., Mutch E., Wright P., James O. F., Rawlins M. D. The effect of alcoholic cirrhosis on the activities of microsomal aldrin epoxidase, 7-ethoxycoumarin O-de-ethylase and epoxide hydrolase, and on the concentrations of reduced glutathione in human liver. Br J Clin Pharmacol. 1983 Jun;15(6):667–672. doi: 10.1111/j.1365-2125.1983.tb01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse K. W., Williams F. M., Mutch E., Wright P., James O. F., Rawlins M. D. The effect of alcoholic cirrhosis on the two kinetic components (high and low affinity) of the microsomal 0-deethylation of 7-ethoxycoumarin in human liver. Eur J Clin Pharmacol. 1984;26(1):61–64. doi: 10.1007/BF00546710. [DOI] [PubMed] [Google Scholar]


