Abstract
Intranasal immunization of mice with a chimeric VP6 protein and the mucosal adjuvant Escherichia coli heat labile toxin LT(R192G) induces nearly complete protection against murine rotavirus (strain EDIM [epizootic diarrhea of infant mice virus]) shedding for at least 1 year. The aim of this study was to identify the protective lymphocytes elicited by this new vaccine candidate. Immunization of mouse strains lacking one or more lymphocyte populations revealed that protection was dependent on αβ T cells but mice lacking γδ T cells and B cells remained fully protected. Furthermore, depletion of CD8 T cells in immunized B-cell-deficient mice before challenge resulted in no loss of protection, while depletion of CD4 T cells caused complete loss of protection. Therefore, αβ CD4 T cells appeared to be the only lymphocytes required for protection. As confirmation, purified splenic T cells from immunized mice were intraperitoneally injected into Rag-2 mice chronically infected with EDIM. Transfer of 2 × 106 CD8 T cells had no effect on shedding, while transfer of 2 × 105 CD4 T cells fully resolved shedding in 7 days. Interestingly, transfer of naive splenic CD4 T cells also resolved shedding but more time and cells were required. Together, these results establish CD4 T cells as effectors of protection against rotavirus after intranasal immunization of mice with VP6 and LT(R192G).
Rotaviruses are the primary cause of severe gastroenteritis in young children and are estimated to be responsible for nearly 50% of the hospitalizations due to diarrhea in this age group in the United States (17). Worldwide, rotaviruses are a major cause of mortality and are estimated to kill approximately 500,000 children annually (U. D. Parashar, E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. G. Glass, Abstr. Conf. on Vaccines for Enteric Diseases, 2001). Vaccines to prevent rotavirus disease evaluated in human trials over the past 20 years have all been live attenuated rotaviruses that are administered orally to mimic natural infection. The protection levels against subsequent rotavirus disease elicited by these vaccines have been highly variable, and none have stimulated complete protection (3). Many of these vaccine candidates have associated side effects, some of which are typical of mild rotavirus illnesses. Others, however, appear to be atypical, the most dramatic of which was the increased risk of intussusception detected after administration of the only licensed rotavirus vaccine, the tetravalent rhesus rotavirus vaccine (31). Because of this, the tetravalent rhesus rotavirus was withdrawn from the U.S. market, and no rotavirus vaccine is currently available for routine administration.
Because of the known and potential deficiencies of live rotavirus vaccines, nonliving rotavirus vaccine candidates have been developed and evaluated in animal models. One candidate is the rotavirus VP6 protein, the structural protein that constitutes the intermediate capsid layer of the rotavirus particle. Intranasal (i.n.) immunization of mice with a chimera of murine rotavirus (strain EDIM [epizootic diarrhea of infant mice virus]) VP6 expressed in Escherichia coli along with a genetically attenuated form of E. coli heat labile toxin [LT(R192G)] as an adjuvant induced nearly complete protection against rotavirus shedding after a subsequent EDIM challenge (6).
Although VP6 is the most immunogenic rotavirus protein, the antibody it stimulates is nonneutralizing. Therefore, the mechanism of protection induced by VP6 immunization is not expected to be a classical viral neutralization. In agreement with this expectation, it was found that protection elicited by i.n. immunization with VP6 and LT(R192G) in immunologically normal mice was retained in B-cell-deficient μMT mice (6). Furthermore, protection against shedding remained intact for at least 1 year following i.n. immunization of mice with VP6 and LT(R192G) (unpublished results). In contrast, immunization of B-cell-deficient mice by oral administration of live murine rotavirus resulted in a gradual loss of protection (11, 27). This indicated that antibody was at least partially responsible for the sterilizing immunity observed in immunologically normal mice after live murine rotavirus immunization. Taken together, these results indicate that the mechanisms of protection induced by oral administration of live rotavirus and i.n. immunization with VP6 plus LT(R192G) are not identical and may be very dissimilar. The purpose of this study is to identify the lymphocytes responsible for protection after i.n. immunization with VP6.
MATERIALS AND METHODS
Virus.
The murine strain of rotavirus (EDIM) used throughout this study was originally obtained from M. Collins (Microbiological Associates, Bethesda, Md.). The pool used to challenge mice after immunization was prepared from the diarrheal stools of neonatal BALB/c mice following oral inoculation with wild-type (i.e., not adapted to growth in cell culture) EDIM. These stools were collected into Earle’s balanced salt solution during the 7 days after rotavirus inoculation and stored at −20°C. The suspensions were later thawed, combined, freon extracted, and centrifuged (1,500 × g, 10 min) to separate phases. The resulting aqueous phase was made into aliquots and stored at −70°C. The rotavirus preparation used in this study had a titer of 2 × 107 focus-forming units/ml as determined for MA104 (monkey kidney) cells by methods described previously (18). Based on an infectivity study in adult mice, this represented a 50% shedding dose (SD50) of 3.6 × 104/ml. The EDIM virus was also adapted to grow in cell culture by multiple passages in MA104 (monkey kidney) cells. After the ninth passage, the virus was triply plaque purified, and this preparation was used for the construction of a recombinant plasmid containing the VP6 gene of EDIM.
Construction of recombinant VP6-encoding plasmid, expression of recombinant VP6 protein, and purification of the expressed VP6 protein.
All steps involving the construction of the pMAL-c2X plasmid containing the EDIM VP6 gene, the expression of the VP6 protein in E. coli as a chimera with maltose-binding protein (MBP), and the purification of the expressed chimeric MBP::VP6 protein have been described in detail elsewhere (6).
MAbs used for staining of isolated lymphocytes.
To stain isolated lymphocytes for analysis by fluorescence-activated cell sorting (FACS), the following monoclonal antibodies (MAbs) were obtained from BD PharMingen (La Jolla, Calif.). B cells were stained with R-phycoerythrin (R-PE)-conjugated anti-mouse immunoglobulin κ chain [Ig(κ)] (MAb 187.1) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD45R/B220 (MAb RA3-6B2). T cells were stained with R-PE-conjugated anti-mouse T-cell receptor (TCR) β chain (MAb H57-597) and either FITC-conjugated anti-mouse CD4 (MAb RM4-4) or FITC-conjugated anti-mouse CD8a (MAb 53-6.7). The MAbs used to stain CD4 T cells and CD8 cells do not compete with the anti-mouse CD4 MAb GK1.5 or anti-mouse CD8 MAb 2.43 used for in vivo depletion, both of which were obtained from the American Type Culture Collection. R-PE-conjugated rat IgG2b (MAb A95-1), R-PE-conjugated rat IgG1 (MAb R3-34), and FITC-conjugated rat IgG2a (MAb R35-95), obtained from BD PharMingen, were used as isotype controls.
Mouse strains.
Inbred mice belonging to haplotypes H-2b and H-2d (i.e., C57BL/6 and BALB/c, respectively) were used in this study. Two strains were used because mice with genetic deficiencies were usually available only on the C57BL/6 background; but immunodeficient (Rag-1) mice on this background shed highly variable and often low amounts of rotavirus antigen after inoculation with wild-type EDIM and were, therefore, not useful for this study. In contrast, immunodeficient mice on a BALB/c background (i.e., Rag-2) chronically and consistently shed large quantities of EDIM after challenge. Since immunologically normal parental strains of both mouse haplotypes also shed large quantities of EDIM following challenge, protection in the immunologically deficient strains could be readily compared to that found in normal mice in each case. The mutant mouse strains used all lacked one or more lymphocyte populations. These included the following: Rag-2 mice with combined B- and T-cell immunodeficiency on a BALB/c background (Taconic, Germantown, N.Y.), nude (thymusless) mice lacking T cells on a C57BL/6 background (Jackson Laboratories, Bar Harbor, Maine), αβ as well as γδ TCR gene knockout mice, both on C57BL/6 backgrounds (Jackson Laboratories), and B-cell-deficient μMt (C57BL/6) and JHD (BALB/c) mice (Jackson Laboratories and Taconic, respectively). All mice were between 6 and 10 weeks of age at the time of their first immunization or viral inoculation. None had been previously infected with rotavirus based on the absence of detectable serum rotavirus IgG. All procedures were carried out in accordance with protocols reviewed and approved by the Children’s Hospital Research Foundation Institutional Animal Care and Use Committee.
Immunization of mice with chimeric VP6.
Groups of mice not previously exposed to rotavirus or rotavirus antigens (i.e., naive) were immunized i.n. under light sedation by administration of 30 μl of immunogen per nostril (9 μg of MBP::VP6) together with 10 μg of attenuated E. coli heat-labile toxin LT(R192G). A second dose was given in the same manner 2 weeks later. Blood specimens were collected by retro-orbital capillary plexus puncture on the day of immunization and 4 weeks after the second immunization to determine the titers of rotavirus antibodies prior to immunization and prior to challenge.
Challenge of mice with EDIM rotavirus and detection of viral shedding.
Four weeks after the second immunization, mice were challenged with 1,000 SD50 of wild-type EDIM by intubation. On the days following challenge, two fecal pellets were collected daily from each mouse and placed into 1.0 ml of Earle’s balanced salt solution. Samples were stored at −20°C and then homogenized and centrifuged (1,500 × g, 5 min) to remove debris before being analyzed for rotavirus antigen. Quantities of rotavirus antigen shed were determined in nanograms per stool specimen by an enzyme-linked immunosorbent assay (ELISA) using methods already described (28).
Determination of rotavirus antibody titers.
Blood samples collected during the study were analyzed for rotavirus IgG by ELISA and expressed in nanograms per milliliter as previously described (28).
In vivo depletion of T-cell populations.
To deplete CD8 or CD4 T cells in naive and VP6-immunized mice prior to EDIM challenge, mice were injected intraperitoneally (i.p.) with 0.2 mg of ammonium sulfate-precipitated MAb beginning 5 days before challenge and administered daily for 4 consecutive days and twice weekly thereafter. The MAbs were obtained from rat hybridoma cell lines 2.43 and GK1.5 against CD8 and CD4 cells, respectively. This depletion schedule consistently resulted in >99% reductions in both splenic and intestinal (mesenteric lymph nodes, intraepithelial lymphocytes [IELs], and lamina propria) CD8 T cells and >85% reductions in CD4 T cells obtained from these sites on the day of challenge as determined by FACS as previously described (27). The same MAbs were used to deplete either CD8 or CD4 T cells in other experiments. Specifically, MAb 2.43 was used to deplete CD8 T cells in Rag-2 mice at the time of adoptive transfer of CD4 T cells, and MAb GK1.5 was used to deplete CD4 T cells in these mice, either at the time of adoptive transfer of CD8 T cells or several weeks after CD4 T-cell transfer. In each case, the injections were administered i.p. with 0.2 mg of MAb per inoculation.
Adoptive transfer of CD4 or CD8 T cells into chronically infected Rag-2 mice.
Donor BALB/c mice either were not immunized or were immunized with two doses of MBP::VP6 and LT(R192G) separated by a 2-week interval as described above. At 4 weeks after the second immunization, the time when these mice would normally be challenged with EDIM, they were sacrificed and their spleens were removed. Single-cell suspensions of spleen cells were made, and red blood cells were lysed with 8.3 mg of ammonium chloride per ml in 0.01 M Tris, pH 7.4. Lymphocytes were washed and resuspended in RPMI medium (Gibco, Inc., Grand Island, N.Y.) containing 10% fetal bovine serum. CD4 or CD8 T cells were first purified by affinity columns specific for each T-cell subtype, performed as prescribed by the manufacturer (R&D Systems, Inc., Minneapolis, Minn.). T-cell purity after use of the columns was 83% for CD4 cells and 86% for CD8 cells. Cells were then stained with MAbs specific for either CD4 or CD8 and sorted twice each on a FACSVantage SE (BD Bioscience, San Jose, Calif.). Purity after sorting was >99% for either CD4 or CD8 T cells. Cells were resuspended in RPMI medium and transferred by i.p. injection into chronically infected Rag-2 mice (recipients). The chronic infection was established by oral inoculation of the mice with 1,000 SD50 of wild-type EDIM. During the period following EDIM inoculation, the infected Rag-2 mice were monitored daily to establish the level of chronic rotavirus antigen shedding. T-cell transfers were conducted between 14 and 28 days after EDIM inoculation of the Rag-2 mice. On the day of and for 2 days after transfer, the mice were depleted of either residual CD4 or CD8 T cells that might still be present after the purification procedures by injection of the appropriate MAb (0.2 mg/injection). After transfer of specified numbers of either CD4 or CD8 T cells, the Rag-2 mice continued to be monitored daily for resolution of EDIM shedding. When shedding appeared to be resolved in certain groups of Rag-2 mice after CD4 T-cell transfer, CD4 cells were depleted by daily i.p. injection (4 consecutive days) of 0.2 mg of MAb GK1.5, and the mice continued to be monitored for EDIM shedding. The quantities of B cells and CD4 and CD8 T cells that populated the spleens, IELs, and lamina propria of the Rag-2 mice were determined by FACS several weeks after transfer.
RESULTS
Protection against rotavirus shedding in VP6-immunized mice requires αβ T cells.
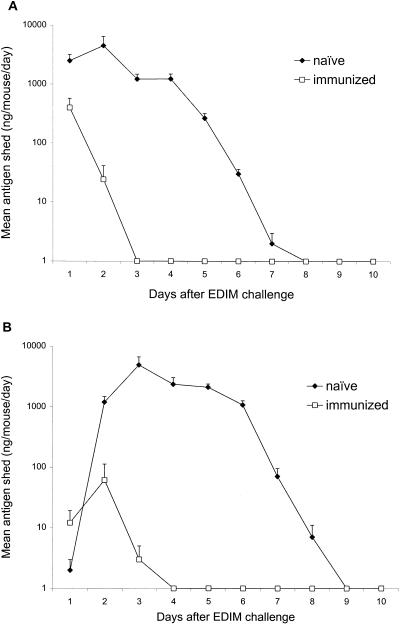
The first approach used to identify which lymphocyte populations are required to elicit protective immune responses in mice following i.n. immunization with VP6 was to immunize mice that had immunological deficiencies due to genetic modifications. Once these mice were immunized, we compared their levels of protection with those stimulated in immunologically normal mice on the same genetic background. When either C57BL/6 or BALB/c mice were challenged with 1,000 SD50 of murine rotavirus strain EDIM, large quantities of rotavirus antigen were shed in their stools during the following 6 to 7 days (Fig. 1A and B). Peak levels of rotavirus antigen shed exceeded 5,000 ng/ml of stool suspension, and average shedding was nearly 1,500 ng/mouse/day during the first 7 days after challenge for both C57BL/6 and BALB/c mice. To determine the effects of VP6 immunization, two doses of MBP::VP6 (9 μg/dose) with 10 μg/dose of LT(R192G) administered i.n. 2 weeks apart consistently reduced rotavirus shedding by >95% in both strains of mice during the week after EDIM challenge, 6 weeks after dose 1 (Fig. 1 and Table 1). In contrast, BALB/c mice with combined immunodeficiency (Rag-2) were totally unprotected after VP6 immunization (Table 1). Likewise, nude (thymusless) C57BL/6 mice and genetically modified C57BL/6 mice lacking αβ TCRs were not significantly protected. However, knockout C57BL/6 mice without γδ TCRs were fully protected by VP6 immunization and no loss of protection was found in B-cell-deficient C57BL/6 (μMT) or BALB/c (JHD) mice. From this it is concluded that αβ T cells are critical for protection but neither γδ T cells nor B cells are required.
FIG. 1.
Effects of i.n. immunization with VP6 and LT(R192G) on protection against shedding of rotavirus antigen in BALB/c (A) or C57BL/6 (B) mice after EDIM challenge. Groups of eight mice were either not immunized or immunized with two doses of antigen and adjuvant (separated by 2 weeks) and challenged with 1,000 SD50 of wild-type EDIM 4 weeks after the second dose. Stools were collected daily and analyzed by ELISA to determine quantities of rotavirus antigen. Error bars represent standard deviations.
TABLE 1.
Levels of protection in different strains of genetically modified mice after VP6 immunizationa
| Mouse strain | Genetic defect | Mean quantity of antigen shed ± SEM (ng)b
|
% Protection from sheddingc | |
|---|---|---|---|---|
| Unimmunized | VP6 immunized | |||
| BALB/c | None | 1,392 ± 325 | 61 ± 24 | 96 |
| C57BL/6 | None | 1,667 ± 323 | 11 ± 7 | 99 |
| BALB/c Rag-2 | Combined immunodeficency | 391 ± 36 | 505 ± 102 | 0 |
| C57BL/6 nude | Thymusless | 22 ± 8 | 20 ± 3 | 9 |
| C57BL/6 Tcrb | No αβ T-cell receptor | 633 ± 72 | 451 ± 49 | 29 |
| C57BL/6 Tcrd | No γδ T-cell receptor | 111 ± 20 | 1 ± 1 | 99 |
| BALB/c JHD | No functional B cells | 3,193 ± 224 | 120 ± 47 | 96 |
| C57BL/6 μMt | No functional B cells | 894 ± 553 | 7 ± 1 | 99 |
Groups of eight mice belonging to the strains specified were either not immunized or were i.n. immunized with two doses of MBP::VP6 and LT(R192G). Four weeks after the second immunization, mice were challenged with 1,000 SD50 of wild-type EDIM, and shedding of rotavirus antigen was monitored for the next 7 days.
Quantity of rotavirus protein/mouse/day for 7 days after EDIM challenge.
Percent protection represents the percentage of decrease in the mean quantity of rotavirus antigen shed due to immunization during the 7 days after challenge.
The αβ T cells required for protection after VP6 immunization of mice belong to the CD4 subset.
We next determined whether CD8, CD4, or both subsets of T cells were required for protection after VP6 immunization. For this, naive mice and mice immunized with VP6 as already described were depleted of either CD8 or CD4 T cells by injection of subtype-specific MAbs beginning 4 days prior to EDIM challenge. This procedure consistently reduced CD8 T cells from both systemic (i.e., spleen) and mucosal (i.e., mesenteric lymph node, IELs, and lamina propria) tissues by >99% and reduced CD4 T-cell levels in these sites by >85%. The mice used in this study belonged to the B-cell-deficient JHD strain to avoid any involvement of B cells.
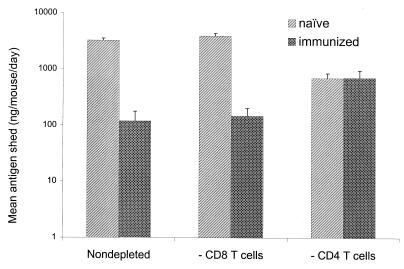
Although depletion of CD8 T cells in naive JHD mice caused high-level, long-term chronic shedding as previously reported (11, 27), this depletion had essentially no effect on EDIM shedding during the first 7 days after challenge, a time during which the nondepleted mice were shedding at a high level (Fig. 2). Furthermore, CD8 T-cell depletion did not reduce the high level of protection stimulated by VP6 immunization of JHD mice, i.e., 96% protection was obtained in both the CD8 cell-depleted and the nondepleted mice. In contrast, CD4 T-cell depletion reduced EDIM shedding in naive JHD mice by ca. 70% during the week after challenge. However, these mice could not fully resolve their infections based on the presence of low-level rotavirus shedding for at least several weeks after challenge, an observation previously found in immunologically normal mice depleted of CD4 T cells (29). When VP6-immunized JHD mice were depleted of CD4 T cells prior to challenge, the level of shedding during the week after challenge was essentially identical to that observed for the unimmunized mice depleted of CD4 T cells (Fig. 2). Also, as found in unimmunized mice, extended low-level shedding was observed (data not shown). These results indicated that the αβ T cells required for protection belonged to the CD4 subtype and CD8 T cells were not needed for protection.
FIG. 2.
Effects of either CD8 or CD4 T-cell depletion on shedding of rotavirus antigen in either naive or VP6-immunized, B-cell-deficient JHD mice during the 7 days after EDIM challenge. Groups of six JHD mice were either not immunized or i.n. immunized with two doses of MBP::VP6 and LT(R192G) separated by 2 weeks. Starting at 24 days after the second dose, some groups of mice were depleted of either CD8 or CD4 T cells by daily (4 consecutive days) injections with MAbs specific for each cell type. On day 28 after the second dose, all mice were challenged with 1,000 SD50 of wild-type EDIM and monitored daily for shedding of rotavirus antigen during the following 7 days. Two additional MAb injections were administered during the 7-day analysis period. The results represent the average amounts in nanograms (ng) of rotavirus antigen shed/mouse/day during the 7-day period, with standard deviations shown by the error bars.
Adoptive transfer of CD4 but not CD8 T cells from either VP6-immunized or naive mice resolves shedding in chronically infected immunodeficient mice.
To establish that CD4 T cells are the only lymphocytes needed to protect against EDIM shedding in VP6-immunized mice, we utilized the chronic shedding model first reported by Riepenhoff-Talty et al. (34) and later used extensively by Greenberg and coworkers (9, 10, 19, 20). Mice with combined B- and T-cell deficiencies, such as Rag-2 mice, become chronically infected after murine rotavirus infection and shed large quantities of virus indefinitely. These mice contain normal amounts of antigen-presenting cells (other than B cells). Therefore, they provide a suitable model with which to determine the lymphocytes needed to resolve a rotavirus infection.
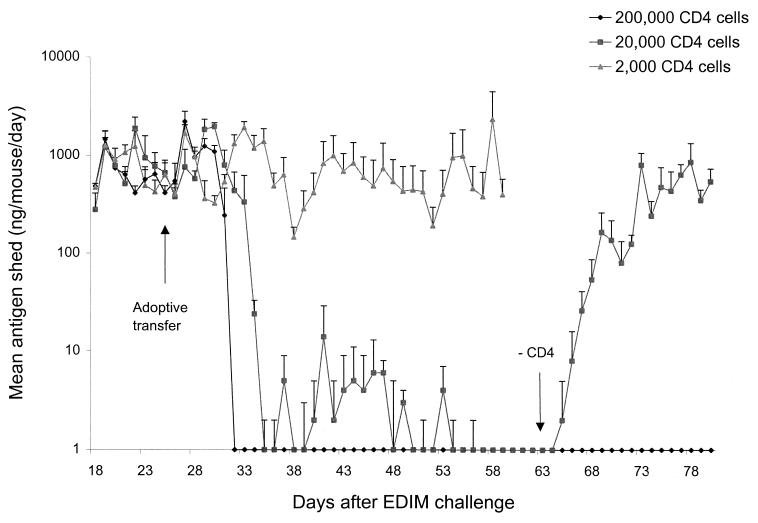
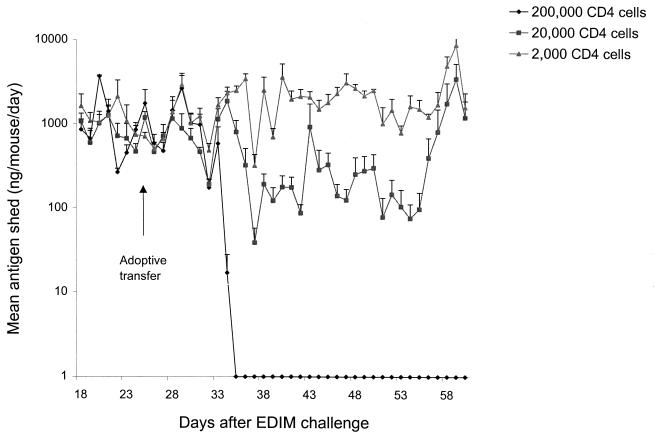
The Rag-2 mice used in this study were on a BALB/c background. Therefore, either splenic CD4 or CD8 T cells from BALB/c mice immunized with MBP::VP6 [two doses of 9 μg with 10 μg of LT(R192G) separated by a 2-week interval] were affinity purified using specific purification columns and doubly sorted (6 weeks after the first immunization) and then transferred by i.p. injection into Rag-2 mice chronically shedding high levels of EDIM. Transfer of as many as 2 × 106 doubly purified, splenic CD8 T cells into the Rag-2 mice caused no reduction in this high level of chronic shedding (data not shown). This occurred even though these cells readily populated and were found in large numbers in the lymphoid tissues (i.e., spleen, IELs, and lamina propria) of the Rag-2 mice at 7 weeks after transfer as determined by FACS analysis (data not shown). On the other hand, transfer of 2 × 105 doubly purified splenic CD4 T cells from VP6-immunized BALB/c mice completely resolved rotavirus shedding in the Rag-2 mice within 7 days (Fig. 3). Furthermore, transfer of 2 × 104 CD4 cells appeared to resolve shedding as well, but over a period of several more days. However, shedding was not completely resolved because depletion of CD4 cells in these mice at 5 weeks after transfer, when shedding was undetectable by ELISA, resulted in the renewal of high-level shedding (Fig. 3). Renewed shedding did not occur when Rag-2 mice that had received 2 × 105 CD4 T cells were depleted of CD4 cells 5 weeks after transfer. Finally, it was found that transfer of 2 × 103 splenic CD4 T cells from VP6-immunized mice had no effect on the high level of chronic rotavirus shedding in the Rag-2 mice (Fig. 3), even though CD4 T cells were readily detected by FACS in the spleens and intestinal tissues of these mice 7 weeks after transfer (data not shown).
FIG. 3.
Rotavirus shedding in Rag-2 mice after adoptive transfer of purified CD4 T cells from VP6-immunized mice. A group of four BALB/c mice were immunized i.n. with two doses of MBP::VP6 and LT(R192G) and 4 weeks after the second dose were sacrificed. Their splenic CD4 T cells were column purified and sorted twice prior to i.p. injection of different quantities into groups of eight Rag-2 mice that were chronically shedding large quantities of rotavirus after oral inoculation of 1,000 SD50 of wild-type EDIM 26 days earlier (day 0). EDIM shedding continued to be monitored daily in all groups until day 60. Starting on day 61, the two groups that received the larger numbers of CD4 T cells were depleted with anti-CD4 MAb injections for 4 consecutive days, and shedding of rotavirus antigen continued to be monitored until day 80.
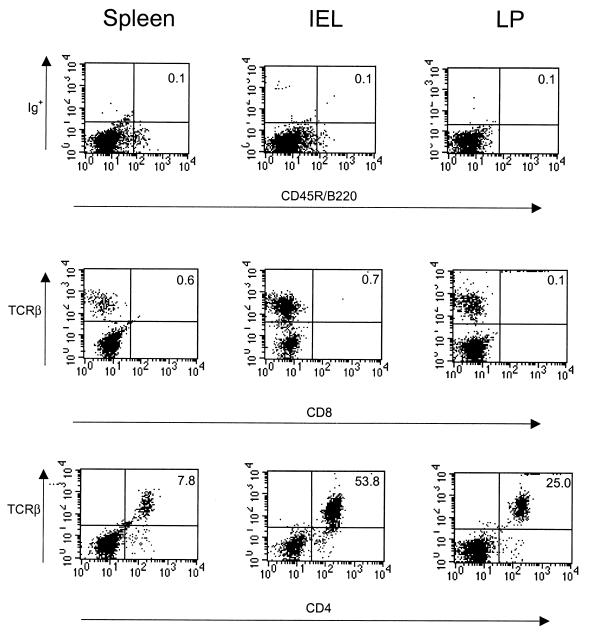
To avoid any possibility that CD8 T cells could be involved in the resolution of shedding, we injected the anti-CD8 T cell MAb 2.43 into the Rag-2 mice at the time of CD4 T-cell transfer. Also, to eliminate the possibility that resolution of shedding was due to other lymphocytes, possibly in conjunction with CD4 T cells, we looked for evidence of B and CD8 T cells in the Rag-2 mice after receiving 2 × 105 CD4 T cells. Seven weeks after CD4 T-cell transfer, we found no evidence of CD8 T cells in either the spleens, IELs, or lamina propria of the Rag-2 mice by FACS analysis (Fig. 4). However, all three sites were populated with large numbers of CD4 T cells. Furthermore, no evidence of B cells was found by FACS (Fig. 4), nor was rotavirus IgG detectable in the sera of the Rag-2 mice by 7 weeks after transfer of 5 × 105 CD4 T cells, 6 weeks after rotavirus shedding had been resolved (data not shown).
FIG. 4.
FACS analysis of either B cells or CD8 or CD4 T cells obtained from the spleens, IELs, or lamina propria of Rag-2 mice 7 weeks after adoptive transfer of purified splenic CD4 T cells from VP6-immunized mice. Splenic CD4 T cells were obtained 4 weeks after the second i.n. immunization of BALB/c mice with MBP::VP6 and LT(R192G), column purified and sorted twice. These cells (2 × 105) were injected i.p. into Rag-2 mice that were chronically shedding large amounts of EDIM. At 7 weeks after transfer, a group of four Rag-2 mice were sacrificed and their splenic and intestinal cells were collected, stained with MAbs against the cell markers specified, and analyzed by FACS (26).
As controls for these studies, we transferred the same number of purified splenic CD4 T cells from naive BALB/c mice into chronically shedding Rag-2 mice. Surprisingly, we observed that 2 × 105 CD4 cells from the naive mice were able to resolve rotavirus shedding although it required an additional 2 days compared to the resolution time found after transfer of 2 × 105 CD4 T cells from VP6-immunized mice (Fig. 5; also Fig. 3). Transfer of 2 × 104 naive CD4 T cells produced only a small, transient reduction in rotavirus shedding, and transfer of 2 × 103 CD4 T cells from naive mice had no effect on shedding. As found after transfer of CD4 T cells from VP6-immunized mice, there was no evidence of either B or CD8 T cells in the Rag-2 mice as determined by FACS analysis of splenic and intestinal cells collected 7 weeks after transfer. Furthermore, there was no evidence of rotavirus antibody production in the Rag-2 mice. Finally, as found when cells were transferred from VP6-immunized mice, transfer of 2 × 106 purified splenic CD8 T cells from naive mice into chronically infected Rag-2 mice readily populated both spleens and intestinal tissues but had no effect on the high level of chronic shedding in these animals.
FIG. 5.
Rotavirus shedding in Rag-2 mice after adoptive transfer of purified CD4 T cells from naive mice. A group of five naive BALB/c mice were sacrificed, and their splenic CD4 T cells were column purified and sorted twice. Specified quantities of these cells were then injected (i.p.) into groups of four Rag-2 mice that were chronically shedding high levels of rotavirus after oral inoculation of 1,000 SD50 of wild-type EDIM 26 days earlier (day 0). Monitoring for rotavirus antigen shedding was continued until day 61.
These results conclusively show that CD4 T cells are the only lymphocytes needed to resolve a rotavirus infection. They also show that CD4 T cells from VP6-immunized mice are more capable of resolving rotavirus infection when transferred alone than are CD8 T cells. Finally, the results suggest that even naive CD4 T cells can be activated when transferred into chronically shedding Rag-2 mice, will traffic to the effector site required to eliminate rotavirus shedding, and will efficiently resolve shedding at this site in the absence of both B and CD8 T cells. Taken together, these results imply that CD4 T cells are the primary effectors of protection after i.n. immunization of mice with MBP::VP6 and LT(R192G).
DISCUSSION
Intranasal immunization of mice with a chimera of the VP6 protein of the murine rotavirus strain EDIM together with the powerful mucosal adjuvant LT(R192G) has been found to consistently stimulate nearly complete protection against rotavirus shedding following a subsequent EDIM challenge. As a result, the VP6 protein is being developed as a possible rotavirus vaccine candidate. In order to optimize the efficacy of a potential VP6 vaccine, it is important to understand the mechanism by which it elicits protection. This study was conducted to identify the lymphocytes responsible for protection after i.n. immunization of mice with expressed VP6 and LT(R192G).
Intranasal VP6 immunization of mice lacking different lymphocyte populations revealed that the presence of T cells with αβ TCRs was a strict requirement for protection. However, the presence of B cells was not required, thus demonstrating that rotavirus antibody was not needed. This contrasts with results found for mice that were orally immunized with live murine rotaviruses (11, 27). In that case, immunized mice lacking B cells were found to rapidly lose their resistance against rotavirus reinfection and subsequent shedding. Live rotavirus immunization results in the production of neutralizing antibody against the VP4 and VP7 outer capsid proteins which can partially explain its dependency on B cells for the sterilizing immunity observed after live virus immunization in immunologically normal mice. VP6 is not a neutralization protein and is, therefore, not expected to elicit production of classical neutralizing antibody. Even so, the presence of circulating monoclonal anti-VP6 IgA in mice protected against viral shedding following oral inoculation with a murine rotavirus (5). This protection was not, however, observed in mice with circulating monoclonal anti-VP6 IgG. Furthermore, i.n. immunization with VP6 and LT(R192G) elicited high titers of serum rotavirus IgG but only very low titers of either serum or stool rotavirus IgA (6). Therefore, the lack of reliance on rotavirus antibody after VP6 immunization was not unexpected.
To determine which αβ T cells were required for protection, VP6-immunized B-cell-deficient JHD mice were depleted of either CD8 or CD4 T cells prior to EDIM challenge and the effects on shedding were measured. CD8 T-cell depletion caused no loss of protection, whereas shedding in mice depleted of CD4 T cells just prior to EDIM challenge was equal for unimmunized and VP6-immunized mice. Thus, protection appeared to depend on CD4 but not CD8 T cells. This conclusion is supported by the finding that a 14-amino-acid peptide within the VP6 protein reported to be a CD4 epitope for H-2d BALB/c mice also stimulated nearly complete protection against EDIM shedding in these mice following i.n. immunization in conjunction with LT(R192G) (7). Furthermore, depletion of CD4 cells in 14-mer-immunized mice prior to EDIM challenge also substantially reduced protection against rotavirus shedding (unpublished results).
Definitive evidence that CD4 T cells in VP6-immunized mice are capable of suppressing rotavirus shedding was found through the chronic shedding model. Immunodeficient Rag-2 mice that were chronically infected with EDIM and were shedding large quantities of rotavirus resolved their infections within 7 days after adoptive transfer of 2 × 105 splenic CD4 T cells from VP6-immunized BALB/c mice. Transfer of 2 × 104 CD4 T cells from the immunized mice reduced shedding below detectable limits (ca. 1,000-fold), but depletion of CD4 cells in these mice resulted in renewed high-level shedding of rotavirus. Therefore, CD4 cells were the only lymphocytes from the immunized mice needed to resolve shedding in chronically infected Rag-2 mice. It was also found that transfer of 2 × 105 splenic CD4 T cells from naive mice resolved shedding in chronically infected Rag-2 mice. However, the time required for resolution was several days longer than needed after transfer of the immune CD4 T cells, an observation with naive cells that was also very recently reported by Kushnir et al. (21). Although other explanations are possible, this result suggested that the CD4 T cells transferred from naive mice were able to traffic to an inductive lymphoid site, become activated after encountering rotavirus antigen, and traffic to the intestinal mucosa where they resolved rotavirus shedding. Since CD4 T cells were the only lymphocytes found in the spleens or intestinal mucosae of the Rag-2 mice several weeks after their transfer from either naive or VP6-immunized mice, it is assumed that no other lymphocytes were needed to resolve the infection.
If CD4 T cells are the only lymphocytes needed to resolve a rotavirus infection, why are B-cell-deficient mice not protected against a subsequent infection after oral immunization with live murine rotaviruses? There are at least three explanations that can be cited for this observed effect. The first is that the shedding which follows reinfection of mice immunized with live rotavirus is very reduced compared to that found after the initial infection (11, 27). This reduction could be entirely due to memory CD8 T cells, as suggested by others (12), or at least partially due to the direct effects of activated CD4 T cells. The importance of the latter cannot be predicted because the role of CD4 T cells in protection against reinfection in B-cell-deficient mice has not been directly investigated. The second explanation comes from several reports showing that development of CD4 T cells requires the help of B cells and mice lacking B cells have impaired CD4 T-cell function (1, 16, 22–24, 35). Our results support this explanation. Adoptive transfer of 106 splenic CD4 T cells from naive JHD (B-cell-deficient) mice into chronically infected Rag-2 mice had no effect on shedding (unpublished results). Furthermore, the transfer of this same number of CD4 T cells from VP6-immunized JHD mice resolved shedding but less efficiently than did the transfer of 2 × 105 CD4 T cells from either naive or VP6-immunized immunologically normal mice. The third explanation is that the roles of CD4 T cells in protection after oral immunization with live rotavirus and i.n. immunization with VP6 and LT(R192G) are very different and that, in fact, these cells have little ability to act as direct effectors of protection after live-virus immunization. The results presented here and in previous reports (11, 27) show that the roles of CD8 T cells and B cells after immunization by the two methods are very different. Therefore, it is not unlikely that the roles of CD4 T cells will also be dissimilar.
It should be noted that adoptive transfer of 2 × 106 splenic CD8 T cells from either naive or VP6-immunized BALB/c mice into chronically infected Rag-2 mice had no effect on rotavirus shedding. It has been reported that CD8 T cells obtained from mice orally immunized with live murine rotavirus are able to resolve rotavirus infection when transferred into chronically infected immunodeficient mice, and the numbers of CD8 T cells required are two- to fourfold less than the amounts we transferred from VP6-immunized mice (10, 19). Investigators from the same laboratory also reported that transfer of 2 × 104 to 3 × 104 CD4 T cells from mice after oral immunization with live murine rotavirus had no effect on shedding (20, 36). These results support the suggestion that the T-cell responses elicited by oral immunization with live rotavirus and i.n. immunization with VP6 and LT(R192G) are very different.
The results reported here provide the first definitive evidence that CD4 T cells can be the only lymphocytes needed to protect against rotavirus, a role previously associated with B and CD8 T cells. Evidence for the direct involvement of CD4 T cells in protection against infection and disease has been previously reported for several viruses, and some studies date back at least 1 decade. In most studies, however, there is only a suggestion that CD4 T cells are the effectors of protection and it is clear that if they are effectors of protection, other effectors still play the major roles. Often, the traditional role of CD4 T cells as helpers for B and CD8 T-cell activation and development has not been eliminated. Even so, there are examples where CD4 T cells may be the only lymphocytes needed for protection. Recent examples include studies with mengovirus (32), Friend virus complex (14), Sendai virus (15, 37), and respiratory syncytial virus (33). Some of the best evidence for direct CD4 T-cell involvement in protection has been found for herpes simplex viruses (8, 25, 26, 30). Even here, however, CD4 T cells have been shown to be only one of the effectors of protection when other lymphocyte subsets are present. Because the immune system is highly redundant, it is possible that in the absence of αβ CD4 T cells, other lymphocyte populations will elicit protection following i.n. immunization with VP6 and LT(R192G). However, our data definitively show that CD4 T cells are the only lymphocytes needed as effectors of protection in mice immunized under these conditions.
The mechanisms by which CD4 T cells protect against rotavirus shedding after i.n. immunization with VP6 has not been examined in this study. In fact, to our knowledge, these mechanisms have not been established for any virus. CD4 T cells have been shown to be cytolytic under certain conditions, and this direct cytotoxic T-lymphocyte activity has been routinely suggested as a possible mechanism (13). In one study with herpes simplex virus type 2 (HSV-2), it was suggested that CD4 memory cells present in the vaginal mucosa of immunized mice may be activated following an HSV-2 reinoculation and recognize processed viral peptides in association with major histocompatibility complex class II proteins on infected vaginal epithelial cells (30). Rotaviruses are known to infect intestinal enterocytes, and these cells can present processed antigen in association with major histocompatibility complex class II proteins (4). Therefore, the same mechanism may be applicable in protection against rotavirus after VP6 immunization. If this is the case, it is also likely that the CD4 memory cells that become activated are retained indefinitely in the intestinal mucosa because no loss of protection was observed even 1 year after i.n. immunization with VP6 and LT(R192G) (unpublished results).
The other mechanism of protection routinely associated with the CD4 T cells is the antiviral activity of the cytokines they produce. Again, there is a scarcity of data that support the direct role of cytokines in protection against viral disease, but there are numerous reports concerning the antiviral activities of cytokines in protection during cell culture infections. A report by Bass demonstrated that gamma interferon (IFN-γ), interleukin 1, and IFN-α all inhibited rotavirus replication in cultured human intestinal cells (2). IFN-γ was also suggested to be the primary CD4 T-cell product associated with protection against HSV-2 replication in vaginal mucosa of mice (8, 30). This cytokine may also be partially responsible for protection against rotavirus shedding after VP6 immunization, but if this is the case, it is only one effector of protection because genetically altered mice lacking IFN-γ remained fully protected after i.n. immunization with VP6 and LT(R192G) (unpublished results).
The protection elicited after VP6 immunization in this study was highly dependent on the presence of the adjuvant. In its absence, no significant protection was observed. Therefore, it is assumed that if VP6 elicits protection against rotavirus shedding in humans after i.n. immunization, this protection will also be dependent on coadministration of a potent mucosal adjuvant. Based on the crucial role of CD4 T cells in protection in this study, it is also assumed that the LT(R192G) adjuvant was instrumental in stimulating production of these protective cells. Although the mechanism by which LT(R193G) boosts immune responses is not fully established, it is known to be a potent stimulator of both Th-1 and Th-2 cell production (28). Knowledge of which CD4 T-cell population plays the more important role in protection will be important to establish, and a vaccination protocol that increases stimulation of this Th subset will be an immediate goal in the development of a VP6 vaccine.
Acknowledgments
We thank Daniel Marmer of Children’s Hospital Medical Center, Cincinnati, Ohio, for his excellent assistance and advice during FACS analyses and sorting of lymphocyte populations.
This work was supported in part by NIH NIAID contract N01 AI-45242 to Children’s Hospital Medical Center.
REFERENCES
- 1.Arulanandam, B. P., R. H. Raeder, J. G. Nedrud, D. J. Bucher, J. Le, and D. W. Metzger. 2001. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 166:226–231. [DOI] [PubMed] [Google Scholar]
- 2.Bass, D. M. 1997. Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology 113:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein, D. I., and R. L. Ward. 1998. Rotaviruses, p1901–1921. In R. D. Feigin and J. D. Cherry (ed.), Textbook of pediatric infectious disease, 4th ed. W. B. Saunders, Philadelphia, Pa.
- 4.Blumberg, R. S., W. I. Lencer, X. Zhu, H.-S. Kim, S. Claypool, S. P. Balk, L. J. Saubermann, and S. P. Colgan. 1999. Antigen presentation by intestinal epithelial cells. Immunol. Lett. 69:7–11. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104–107. [DOI] [PubMed] [Google Scholar]
- 6.Choi, A. H.-C., M. Basu, M. M. McNeal, J. D. Clements, and R. L. Ward. 1999. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J. Virol. 73:7574–7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, A. H.-C., M. Basu, M. M. McNeal, J. Flint, J. L. VanCott, J. D. Clements, and R. L. Ward. 2000. Functional mapping of protective domains and epitopes in the rotavirus VP6 protein. J. Virol. 74:11574–11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4+ T cell-mediated control of a γ-herpesvirus in B cell-deficient mice is mediated by IFN-γ. Proc. Natl. Acad. Sci. USA 96:5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharakul, T., M. Labbe, J. Cohen, A. R. Bellamy, J. E. Street, E. R. Mackow, L. Fiore, L. Rott, and H. B. Greenberg. 1991. Immunization with baculovirus-expressed recombinant rotavirus proteins VP1, VP4, VP6, and VP7 induces CD8+ T lymphocytes that mediate clearance of chronic rotavirus infection in SCID mice. J. Virol. 65:5928–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharakul, T., L. Rott, and H. B. Greenberg. 1990. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency: virus clearance mediated by adoptive transfer of immune CD8+ T lymphocytes. J. Virol. 64:4375–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco, M. A., and H. B. Greenberg. 1995. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 69:7800–7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco, M. A., C. Tin, and H. B. Greenberg. 1997. CD8+ T cells can mediate almost complete short-term and partial long-term immunity to rotavirus in mice. J. Virol. 71:4165–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn, S., R. Gehri, and P. Erb. 1995. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol. Rev. 146:57–79. [DOI] [PubMed] [Google Scholar]
- 14.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan, R. J., W. Zhong, E. J. Usherwood, T. Cookenham, A. D. Roberts, and D. L. Woodland. 2001. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J. Exp. Med. 193:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homann, D., A. Tishon, D. P. Berger, W. O. Weigle, M. G. von Herrath, and M. B. A. Oldstone. 1998. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from μMT/μMT mice. J. Virol. 72:9208–9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapikian, A. Z., and R. M. Chanock. 1990. Rotaviruses, p.1353–1404. In B. N. Fields, D. M. Knipe, P. M. Howley, R.M Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology, 2nd ed. Raven Press, New York, N.Y.
- 18.Knowlton, D. R., D. M. Spector, and R. L. Ward. 1991. Development of an improved method for measuring neutralizing antibody to rotavirus. J. Virol. Methods 33:127–134. [DOI] [PubMed] [Google Scholar]
- 19.Kuklin, N. A., L. Rott, J. Darling, J. J. Campbell, M. Franco, N. Feng, W. Muller, N. Wagner, J. Altman, E. C. Butcher, and H. B. Greenberg. 2000. α4β7 independent pathway for CD8+ T cell-mediated intestinal immunity to rotavirus. J. Clin. Investig. 106:1541–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuklin, N. A., L. Rott, N. Feng, M. C. Conner, N. Wagner, W. Muller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B cell immunity is dependent on α4β7 integrin expression but does not require IgA antibody production. J. Immunol. 166:1894–1902. [DOI] [PubMed] [Google Scholar]
- 21.Kushnir, N., N. A. Bos, A. W. Zuercher, S. E. Coffin, C. A. Moser, P. A. Offit, and J. J. Cebra. 2001. B2 but not B1 cells can contribute to CD4+ T-cell-mediated clearance of rotavirus in SCID mice. J. Virol. 75:5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linton, P.-J., J. Harbertson, and L. M. Bradley. 2000. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 165:5558–5565. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Y., Y. Wu, L. Ramarathinam, Y. Guo, D. Huszar, M. Trounstine, and M. Zhao. 1995. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int. Immunol. 7:1353–1362. [DOI] [PubMed] [Google Scholar]
- 24.Macaulay, A. E., R. H. DeKruyff, D. T. Umetsu. 1998. Antigen-primed T cells from B cell-deficient JHD mice fail to provide B cell help. J. Immunol. 160:1694–1700. [PubMed] [Google Scholar]
- 25.Manickan, E., M. Francotte, N. Kuklin, M. Dewerchim, C. Molitor, D. Gheysen, M. Slaoui, and B. T. Rouse. 1995. Vaccination with recombinant vaccinia viruses expressing ICP27 induces protective immunity against herpes simplex virus through CD4+ Th1+ T cells. J. Virol. 69:4711–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manickan, E., R. J. D. Rouse, Z. Yu, W. S. Wire, and B. T. Rouse. 1995. Genetic immunization against herpes simplex virus. J. Immunol. 155:259–265. [PubMed] [Google Scholar]
- 27.McNeal, M. M., K. S. Barone, M. N. Rae, and R. L. Ward. 1995. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology 214:387–397. [DOI] [PubMed] [Google Scholar]
- 28.McNeal, M. M., M. N. Rae, J. A. Bean, and R. L. Ward. 1999. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J. Virol. 73:7565–7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeal, M. M., M. N. Rae, and R. L. Ward. 1997. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J. Virol. 71:8735–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093–6100. [PubMed] [Google Scholar]
- 31.Murphy, T. V., P. M. Gargiullo, M. S. Massoudi, D. B. Nelson, A. O. Jumaan, C. A. Okoro, L. R. Zanardi, A. Setia, E. Fair, C. W. LeBaron, R. Schwartz, M. Wharton, and J. R. Livingood. 2001. Intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 344:564–572. [DOI] [PubMed] [Google Scholar]
- 32.Neal, Z. C., and G. A. Splitter. 1995. Picornavirus-specific CD4+ T lymphocytes possessing cytolytic activity confer protection in the absence of prophylactic antibodies. J. Virol. 69:4914–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plotnicky-Gilquin, H., A. Robert, L. Chevalet, J.-F. Haeuw, A. Beck, J.-Y. Bonnefoy, C. Brandt, C.-A. Siegrist, T. Ngoc Nguyen, and U. F. Power. 2000. CD4+ T-cell-mediated antiviral protection of the upper respiratory tract in BALB/c mice following parenteral immunization with a recombinant respiratory syncytial virus G protein fragment. J. Virol. 74:3455–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riepenhoff-Talty, M., T. Dharakul, E. Kowalski, S. Michalak, and P. L. Ogra. 1987. Persistent rotavirus infection in mice with severe combined immunodeficiency. J. Virol. 61:3345–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Essen, D., P. Dullforce, T. Brocker, and D. Gray. 2000. Cellular interactions involved in Th cell memory. J. Immunol. 165:3640–3646. [DOI] [PubMed] [Google Scholar]
- 36.Williams, M. B., J. R. Rosé, L. S. Rott, M. A. Franco, H. B. Greenberg, and E. C. Butcher. 1998. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, α4β7. J. Immunol. 161:4227–4235. [PubMed] [Google Scholar]
- 37.Zhong, W., D. Marshall, C. Coleclough, and D. L. Woodland. 2000. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J. Immunol. 164:3274–3282. [DOI] [PubMed] [Google Scholar]