Abstract
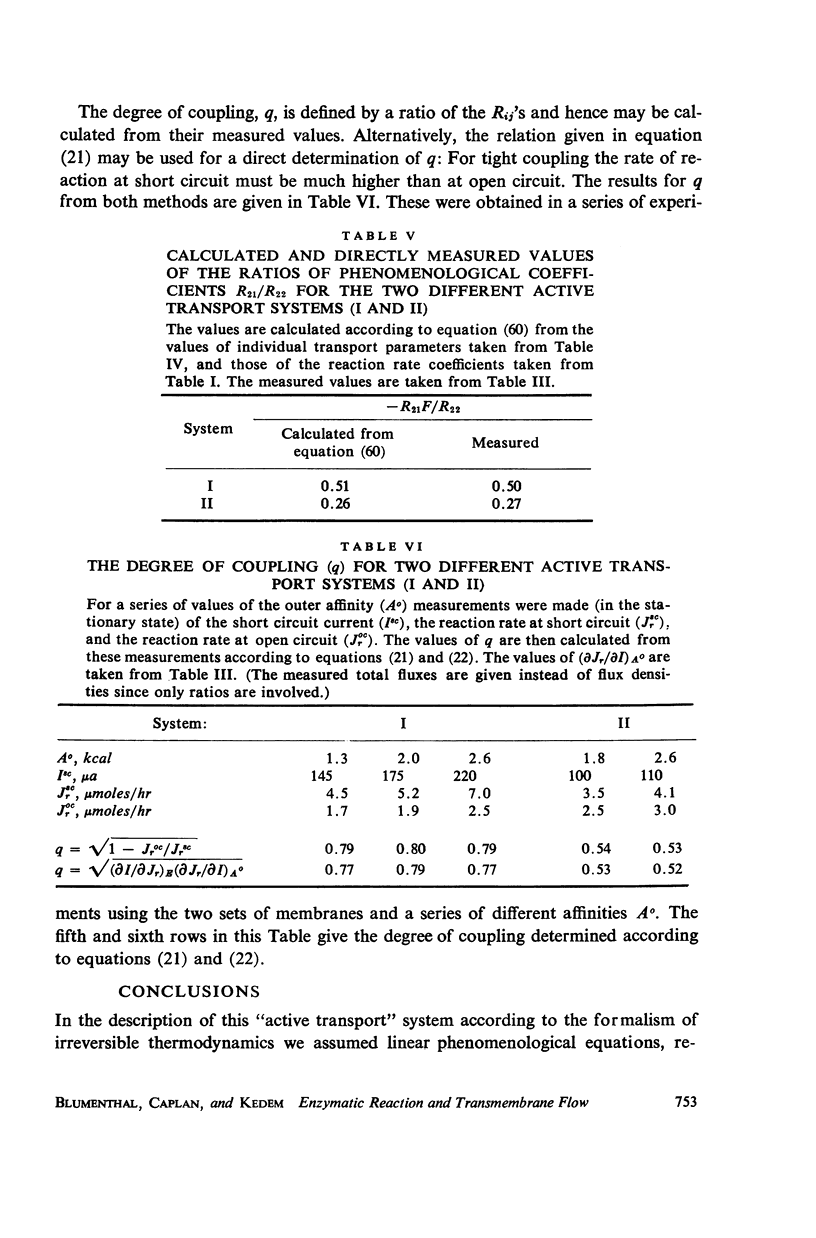
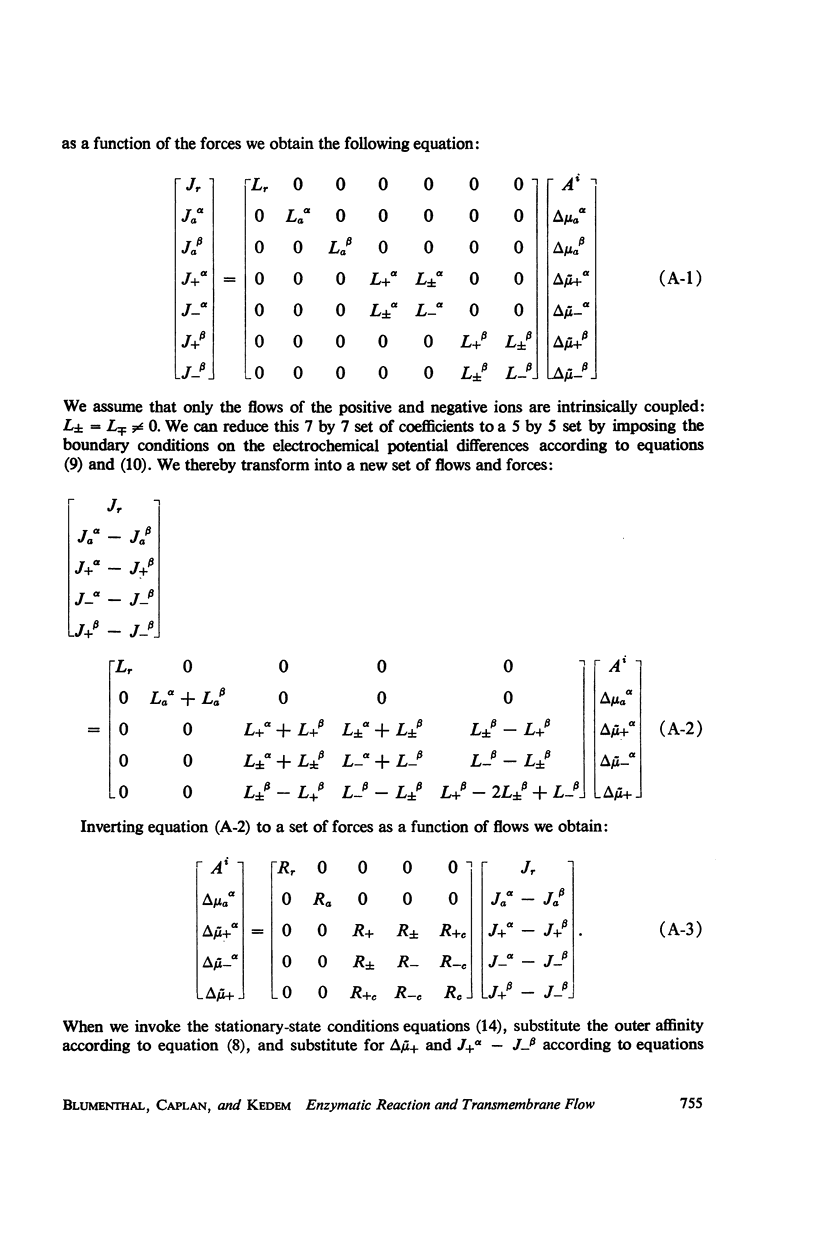
If a chemical reaction is constrained to occur within an asymmetric structure, e.g. by the presence of bound or otherwise trapped enzyme, coupling of the reaction to the flow of one or more solutes, or to the flow of electric current, becomes possible. Such systems can serve as models in which transport is “driven” by chemical reaction. In this respect the processes involved are analogous to active transport, though the molecular mechanisms may be quite different from those in nature. A simple arrangement of this kind has been studied: a composite membrane consisting of two ion exchange membranes of opposite fixed charge, separated by an intermediate layer of solution containing papain. An uncharged substrate of low molecular weight acts as “fuel” for the system, N-acetyl-L-glutamic acid diamide. This material (not previously described) hydrolyzes in the presence of papain to ammonium N-acetyl-L-glutamine. The composite membrane gives rise to an electromotive force, ultimately reaching a stationary state, when clamped between two identical solutions in which the affinity of the reaction has been fixed. Onsager's reciprocity relation has not hitherto been tested in a case of coupling between chemical reaction and a vectorial flow (here electric current); its validity for this system, in which stationary-state coupling occurs, was established over the experimental range of affinities (up to 3 kcal/mole).
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caplan S. R. A characteristic of self-regulated linear energy converters. The Hill force-velocity relation for muscle. J Theor Biol. 1966 May;11(1):63–86. doi: 10.1016/0022-5193(66)90040-3. [DOI] [PubMed] [Google Scholar]


