Abstract
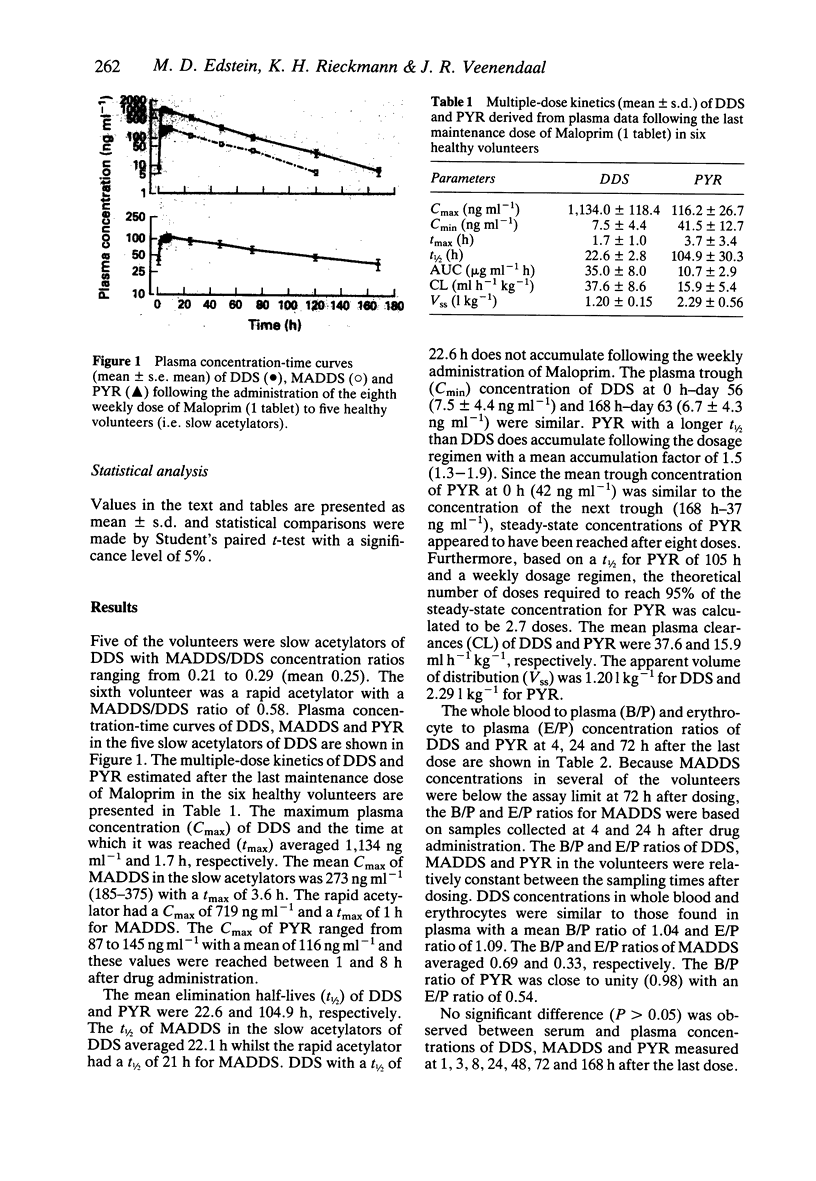
1. The multiple-dose kinetics of dapsone (DDS), its major metabolite monoacetyldapsone (MADDS) and pyrimethamine (PYR) were studied in six healthy adult male volunteers following weekly administration of Maloprim (100 mg DDS plus 12.5 mg PYR). 2. After the last maintenance dose of Maloprim, the following kinetic parameters (mean values) were determined for DDS and PYR, respectively: maximum plasma concentration (Cmax) = 1,134 and 116 ng ml-1; elimination half-life (t1/2) = 23 and 105 h; plasma clearance (CL) = 37.6 and 15.9 ml h-1 kg-1 and apparent volume of distribution (Vss) = 1.20 and 2.29 l kg-1. The mean t1/2 of MADDS was 22 h. 3. The mean whole blood to plasma (B/P) and erythrocyte to plasma (E/P) concentration ratios for DDS were 1.04 and 1.09, respectively. MADDS had a B/P ratio of 0.69 and an E/P ratio of 0.33. The B/P and E/P ratios for PYR were 0.98 and 0.54, respectively. 4. The drug combination was assessed in vitro by measuring inhibition of re-invasion of two Plasmodium falciparum isolates grown in the presence of volunteers' sera. The chloroquine (CQ)- and PYR-sensitive FC-27 isolate was completely inhibited by the sera but the drug combination was ineffective against the CQ- and PYR-resistant K1 strain. The in vitro findings suggest that Maloprim may not be effective against strains of P. falciparum with a high level of resistance to pyrimethamine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad R. A., Rogers H. J. Pharmacokinetics and protein binding interactions of dapsone and pyrimethamine. Br J Clin Pharmacol. 1980 Nov;10(5):519–524. doi: 10.1111/j.1365-2125.1980.tb01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist Y., Domeij-Nyberg B. Distribution of chloroquine and its metabolite desethyl-chloroquine in human blood cells and its implication for the quantitative determination of these compounds in serum and plasma. J Chromatogr. 1983 Jan 14;272(1):137–148. doi: 10.1016/s0378-4347(00)86110-1. [DOI] [PubMed] [Google Scholar]
- Biddulph J. Malaria chemoprophylaxis: an update. P N G Med J. 1987 Mar;30(1):53–56. [PubMed] [Google Scholar]
- Edstein M. D. Pharmacokinetics of sulfadoxine and pyrimethamine after Fansidar administration in man. Chemotherapy. 1987;33(4):229–233. doi: 10.1159/000238499. [DOI] [PubMed] [Google Scholar]
- Gelber R., Peters J. H., Gordon G. R., Glazko A. J., Levy L. The polymorphic acetylation of dapsone in man. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):225–238. doi: 10.1002/cpt1971122part1225. [DOI] [PubMed] [Google Scholar]
- Jones C. R., Ovenell S. M. Determination of plasma concentrations of dapsone, monoacetyl dapsone and pyrimethamine in human subjects dosed with maloprim. J Chromatogr. 1979 Jun 11;163(2):179–185. doi: 10.1016/s0378-4347(00)81461-9. [DOI] [PubMed] [Google Scholar]
- Lamont G., Darlow B. Comparison of in vitro pyrimethamine assays and in vivo response to sulphadoxine-pyrimethamine in Plasmodium falciparum from Papua New Guinea. Trans R Soc Trop Med Hyg. 1982;76(6):797–799. doi: 10.1016/0035-9203(82)90111-0. [DOI] [PubMed] [Google Scholar]
- Milhous W. K., Weatherly N. F., Bowdre J. H., Desjardins R. E. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob Agents Chemother. 1985 Apr;27(4):525–530. doi: 10.1128/aac.27.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi D. S., Poznanski W. J., Smith F. M. Effects of contact with vacutainer tube stoppers on the estimation of quinidine in serum and plasma. Clin Biochem. 1980 Dec;13(6):297–300. doi: 10.1016/s0009-9120(80)80014-2. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Murray J. F., Jr, Gordon G. R., Gelber R. H. Dapsone in saliva and plasma of man. Pharmacology. 1981;22(3):162–171. doi: 10.1159/000137486. [DOI] [PubMed] [Google Scholar]
- Pieters F. A., Zuidema J. The pharmacokinetics of dapsone after oral administration to healthy volunteers. Br J Clin Pharmacol. 1986 Oct;22(4):491–494. doi: 10.1111/j.1365-2125.1986.tb02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidenberg M. M., Drayer D., DeMarco A. L., Bello C. T. Hydralazine elimination in man. Clin Pharmacol Ther. 1973 Nov-Dec;14(6):970–977. doi: 10.1002/cpt1973146970. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Weidekamm E., Mimica I., Heizmann P., Portmann R. Multiple-dose pharmacokinetics of the antimalarial drug Fansimef (pyrimethamine + sulfadoxine + mefloquine) in healthy subjects. Chemotherapy. 1987;33(1):1–8. doi: 10.1159/000238468. [DOI] [PubMed] [Google Scholar]
- Scott H. V., Edstein M. D., Veenendaal J. R., Rieckmann K. H. A sensitive bioassay for serum cycloguanil using Plasmodium falciparum in vitro. Int J Parasitol. 1988 Jul;18(5):605–609. doi: 10.1016/0020-7519(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Scott H. V., Rieckmann K. H., O'Sullivan W. J. Synergistic antimalarial activity of dapsone/dihydrofolate reductase inhibitors and the interaction of antifol, antipyrimidine and antipurine combinations against Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1987;81(5):715–721. doi: 10.1016/0035-9203(87)90004-6. [DOI] [PubMed] [Google Scholar]
- Spencer H. C., Watkins W. M., Sixsmith D. G., Koech D. K., Chulay J. D. A new in vitro test for pyrimethamine/sulfadoxine susceptibility of Plasmodium falciparum and its correlation with in vivo resistance in Kenya. Bull World Health Organ. 1984;62(4):615–621. [PMC free article] [PubMed] [Google Scholar]
- Spracklen F. H., Monteagudo F. S. Malaria prophylaxis. S Afr Med J. 1986 Sep 13;70(6):316–316. [PubMed] [Google Scholar]
- Stürchler D. Malaria prophylaxis in travellers: the current position. Experientia. 1984 Dec 15;40(12):1357–1362. doi: 10.1007/BF01951889. [DOI] [PubMed] [Google Scholar]
- Weidekamm E., Schwartz D. E., Dubach U. C., Weber B. Single-dose investigation of possible interactions between the components of the antimalarial combination Fansimef. Chemotherapy. 1987;33(4):259–265. doi: 10.1159/000238505. [DOI] [PubMed] [Google Scholar]
- Zuidema J., Hilbers-Modderman E. S., Merkus F. W. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986 Jul-Aug;11(4):299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]


