Abstract
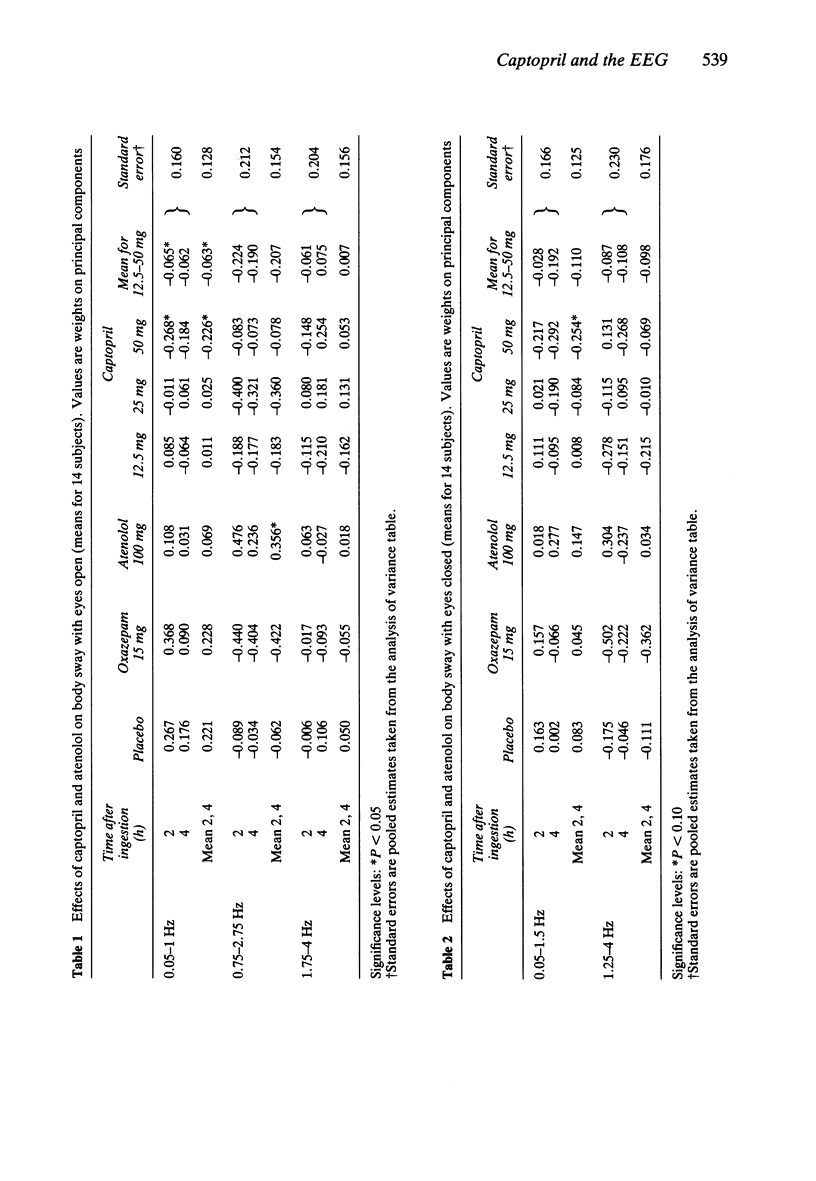
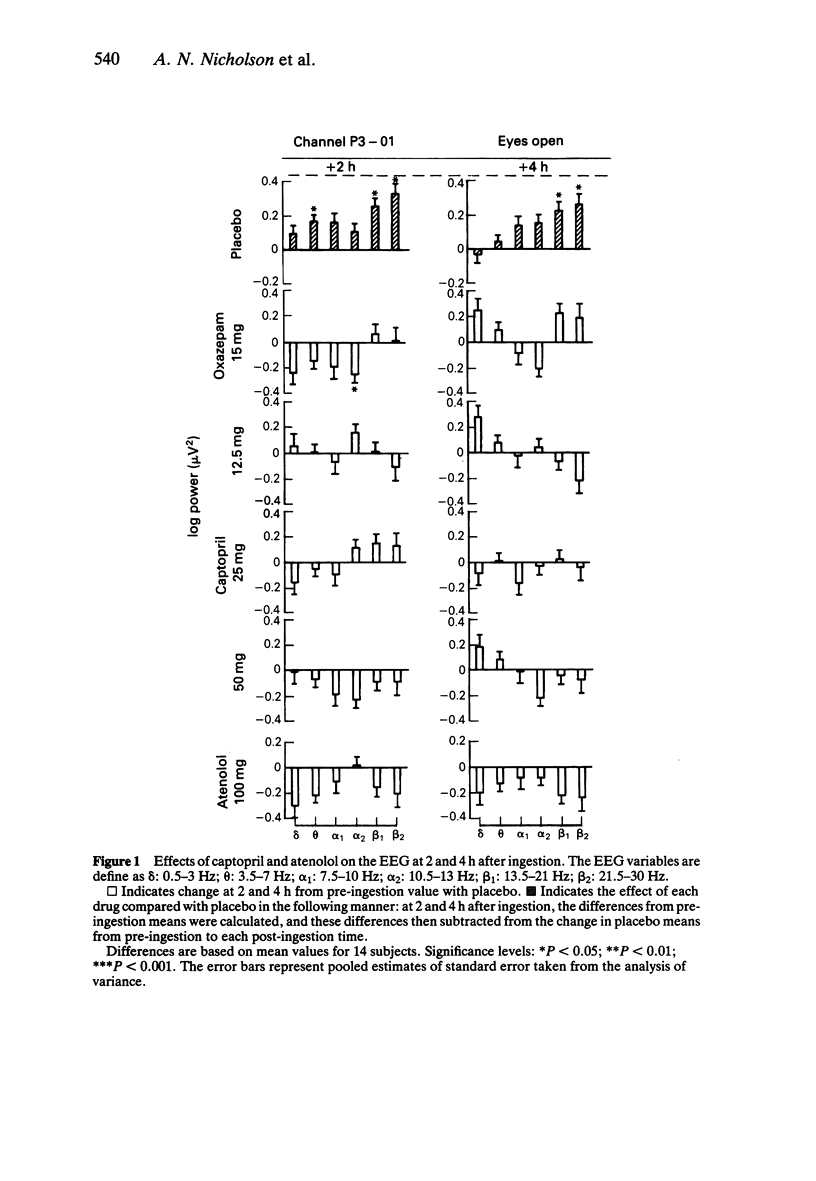
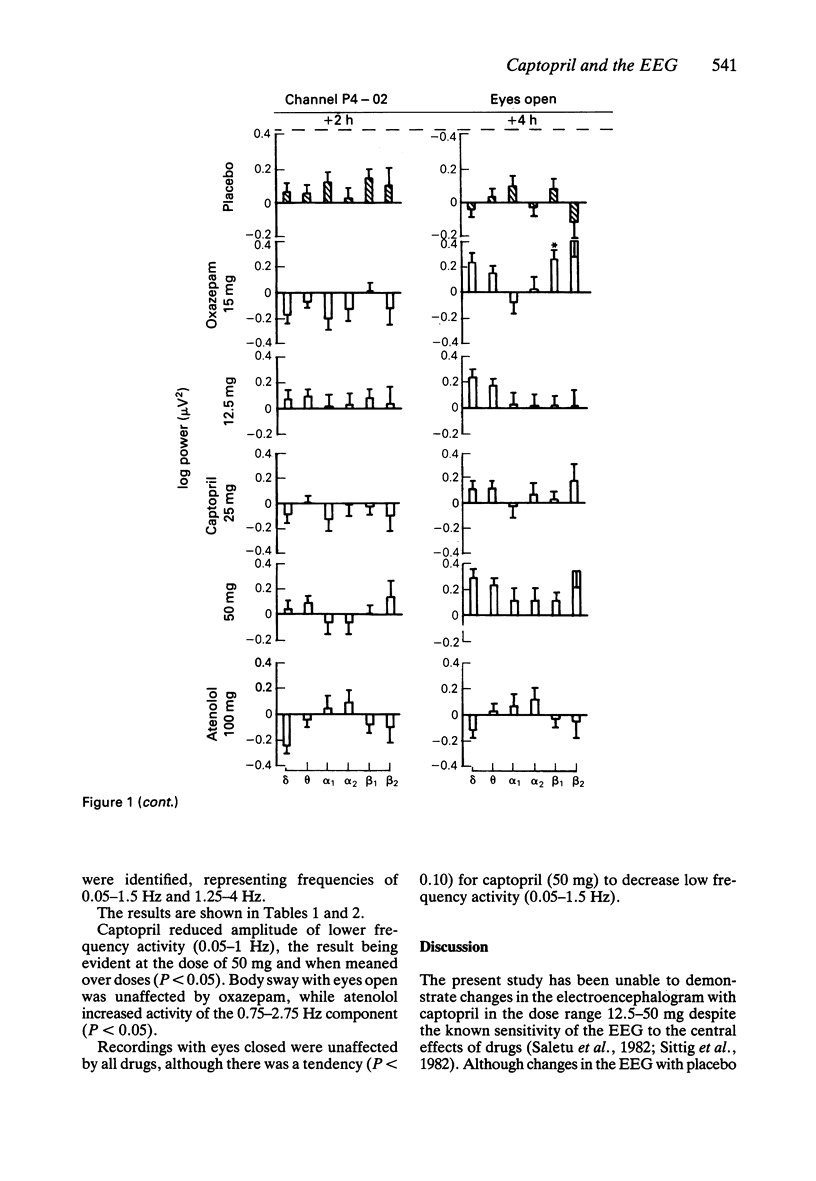
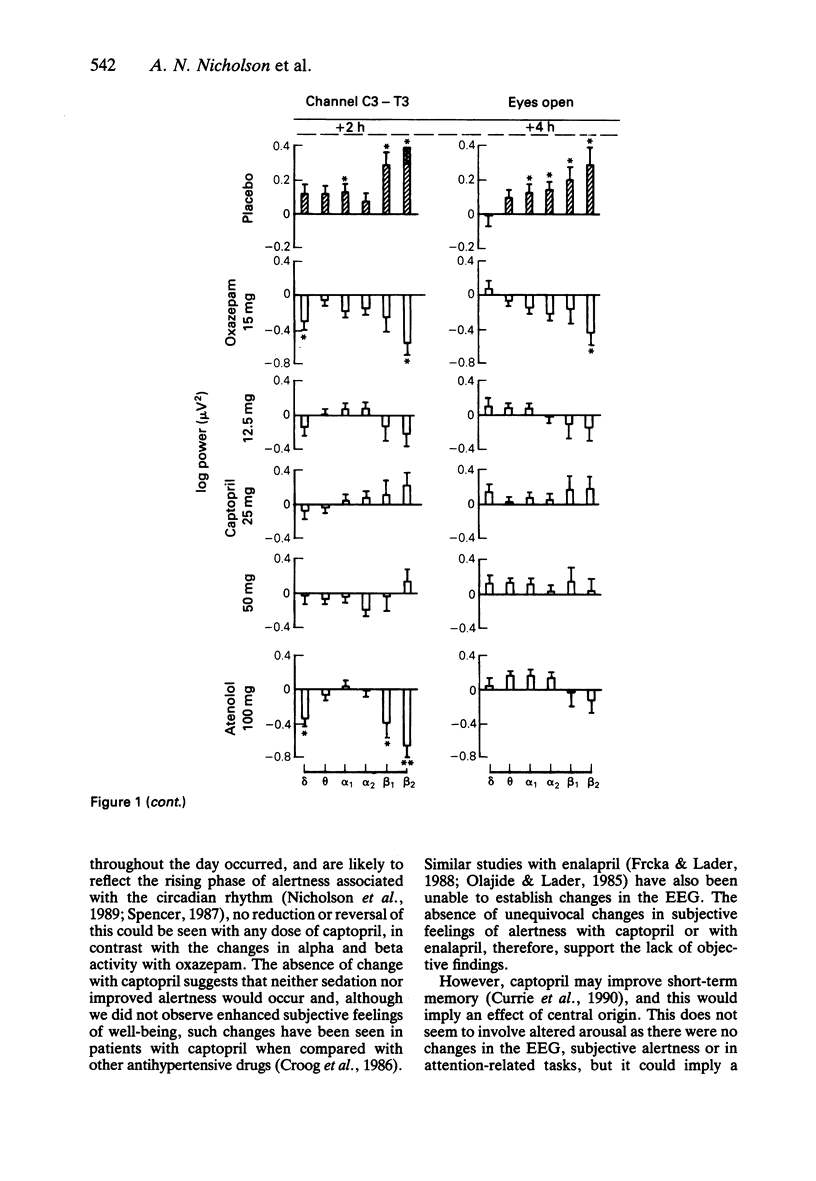
1. Effects of single doses of captopril (12.5, 25 and 50 mg) on the electroencephalogram (EEG) and on body sway were studied in fourteen healthy male subjects. Oxazepam (15 mg), as an active control, and two placebos were included in the study, together with a single dose of atenolol (100 mg). Medication was administered double-blind at 11.00 h, and assessments made before and at 2 and 4 h after drug ingestion. 2. There were no changes in the EEG with captopril. Oxazepam reduced the circadian rise in alpha activity, while atenolol decreased beta power. Delta activity was modified by both oxazepam and atenolol. 3. A reduction in lower frequencies of body sway (0.05-1 Hz) occurred with captopril, while the spectra were unaffected by oxazepam. Atenolol increased (P less than 0.05) activity in the frequency range 0.75-2.75 Hz. 4. These observations suggest that captopril is free of central effects such as sedation that may occur with beta-adrenoceptor antagonists. Reduced body sway with captopril could reflect improved integration of central and peripheral control of posture.
Full text
PDF

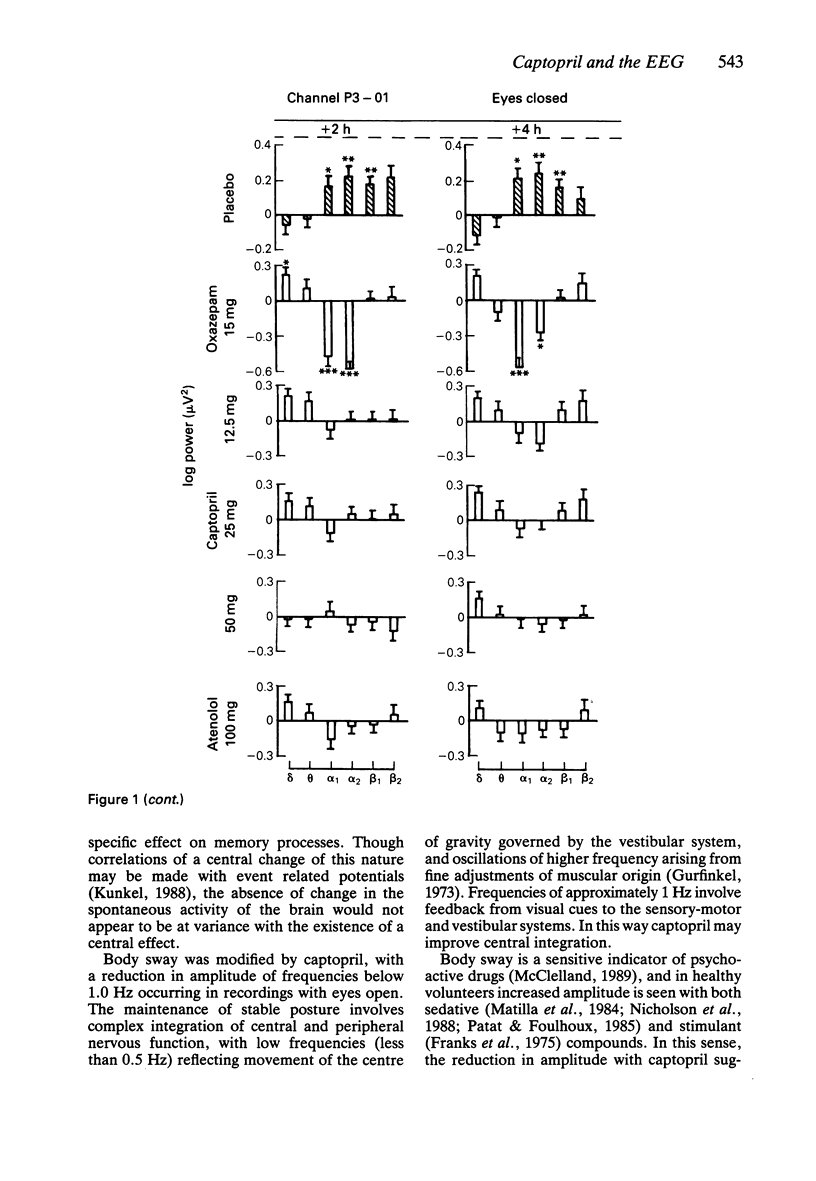
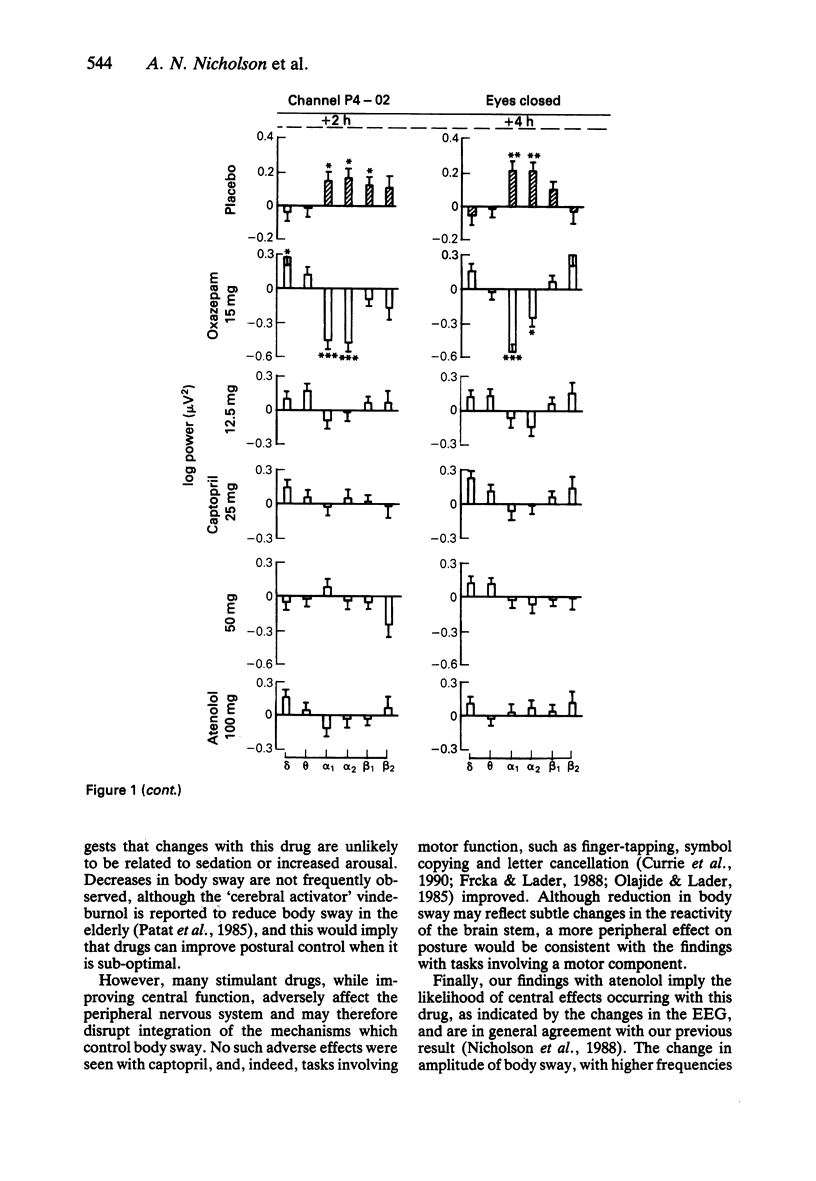
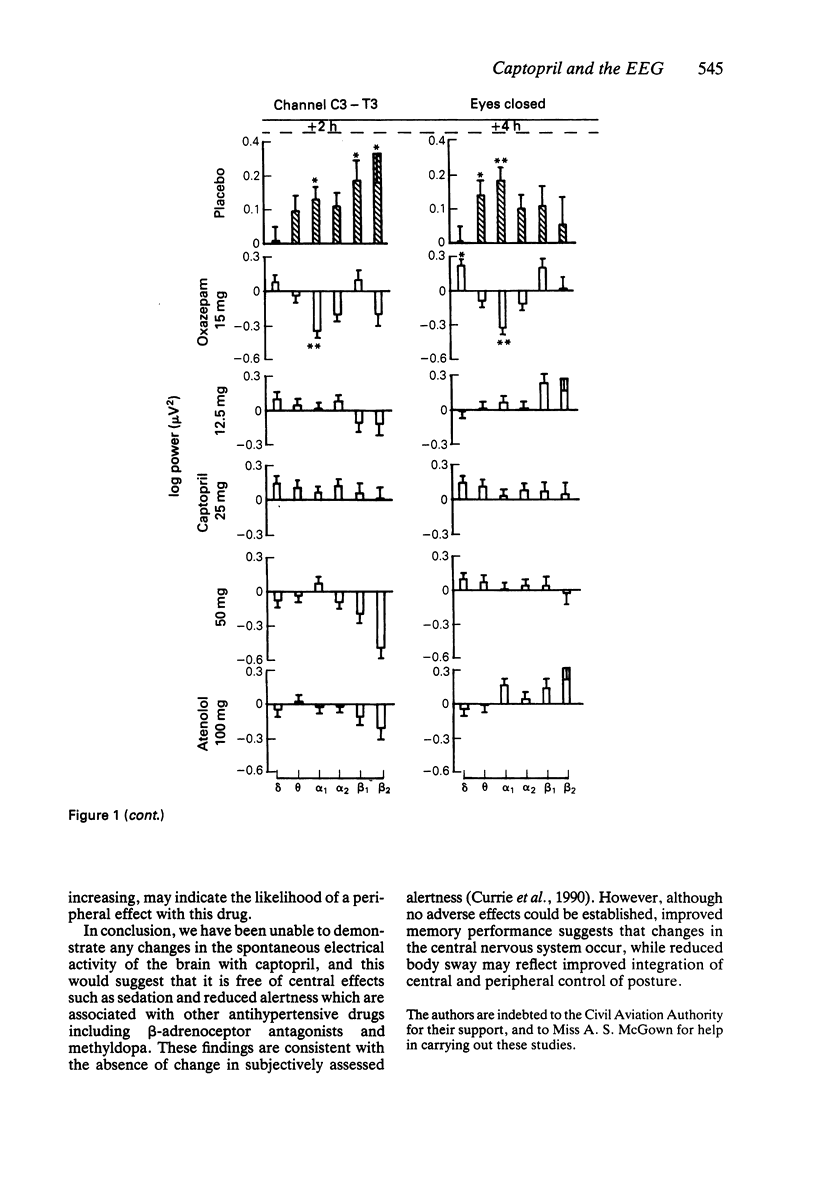

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croog S. H., Levine S., Testa M. A., Brown B., Bulpitt C. J., Jenkins C. D., Klerman G. L., Williams G. H. The effects of antihypertensive therapy on the quality of life. N Engl J Med. 1986 Jun 26;314(26):1657–1664. doi: 10.1056/NEJM198606263142602. [DOI] [PubMed] [Google Scholar]
- Currie D., Lewis R. V., McDevitt D. G., Nicholson A. N., Wright N. A. Central effects of the angiotensin-converting enzyme inhibitor, captopril. I. Performance and subjective assessments of mood. Br J Clin Pharmacol. 1990 Oct;30(4):527–536. doi: 10.1111/j.1365-2125.1990.tb03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks H. M., Hagedorn H., Hensley V. R., Hensley W. J., Starmer G. A. The effect of caffeine on human performance, alone and in combination with ethanol. Psychopharmacologia. 1975 Dec 31;45(2):177–181. doi: 10.1007/BF00429058. [DOI] [PubMed] [Google Scholar]
- Frcka G., Lader M. Psychotropic effects of repeated doses of enalapril, propranolol and atenolol in normal subjects. Br J Clin Pharmacol. 1988 Jan;25(1):67–73. doi: 10.1111/j.1365-2125.1988.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila M. J., Aranko K., Mattila M. E., Strömberg C. Objective and subjective assessment of hangover during subacute administration of temazepam and nitrazepam to healthy subjects. Eur J Clin Pharmacol. 1984;26(3):375–380. doi: 10.1007/BF00548770. [DOI] [PubMed] [Google Scholar]
- Nicholson A. N., Wright N. A., Zetlein M. B., Currie D., McDevitt D. G. Central effects of beta-adrenoceptor antagonists. II--Electroencephalogram and body sway. Br J Clin Pharmacol. 1988 Aug;26(2):129–141. doi: 10.1111/j.1365-2125.1988.tb03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajide D., Lader M. Psychotropic effects of enalapril maleate in normal volunteers. Psychopharmacology (Berl) 1985;86(3):374–376. doi: 10.1007/BF00432232. [DOI] [PubMed] [Google Scholar]
- Patat A., Foulhoux P. Effect on postural sway of various benzodiazepine tranquillizers. Br J Clin Pharmacol. 1985 Jul;20(1):9–16. doi: 10.1111/j.1365-2125.1985.tb02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patat A., Le Go A., Foulhoux P. Dose-response relationship of vindeburnol based on spectral analysis of posturographic recordings. Eur J Clin Pharmacol. 1985;29(4):455–459. doi: 10.1007/BF00613461. [DOI] [PubMed] [Google Scholar]


