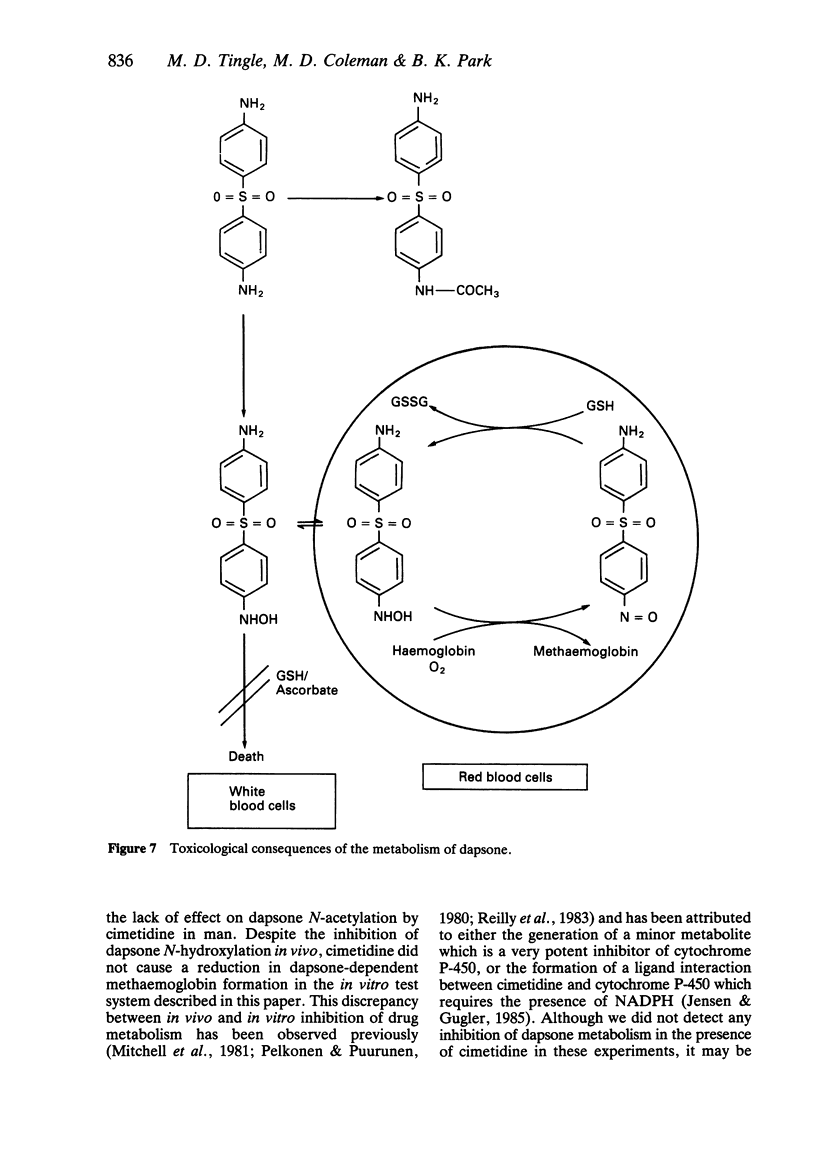
Abstract
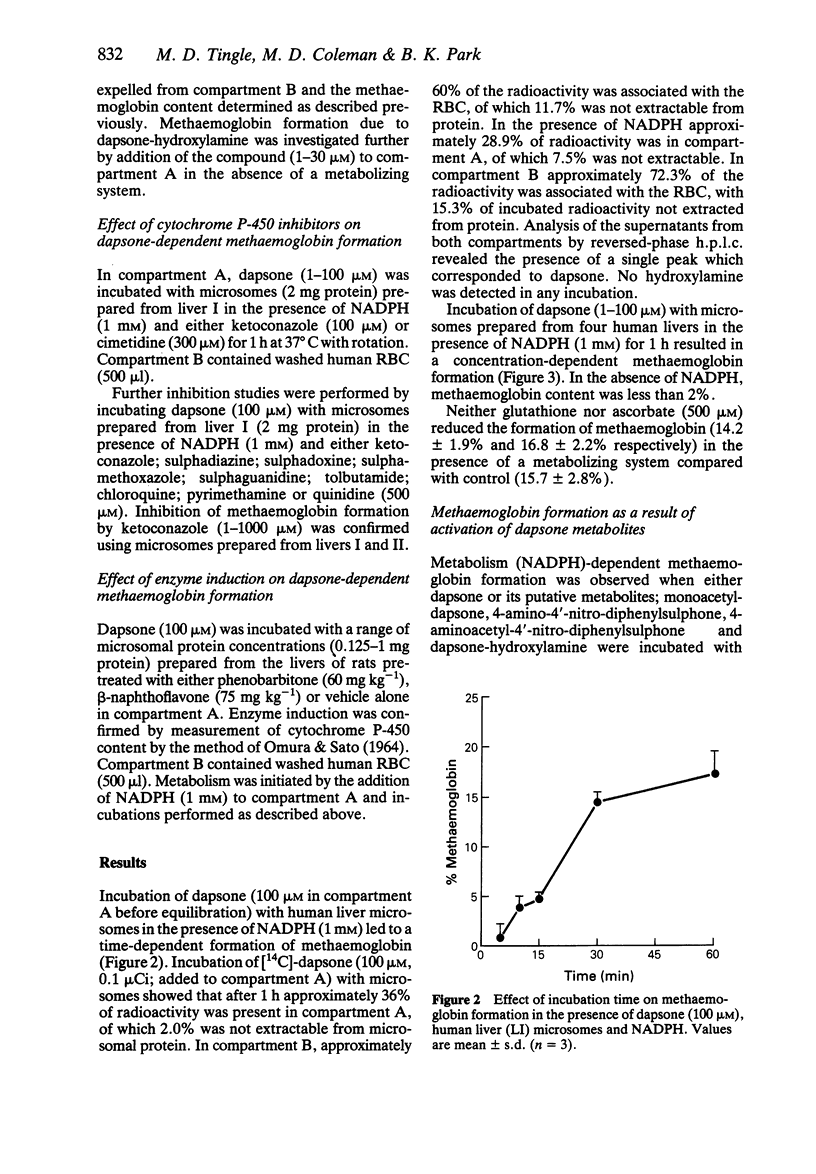
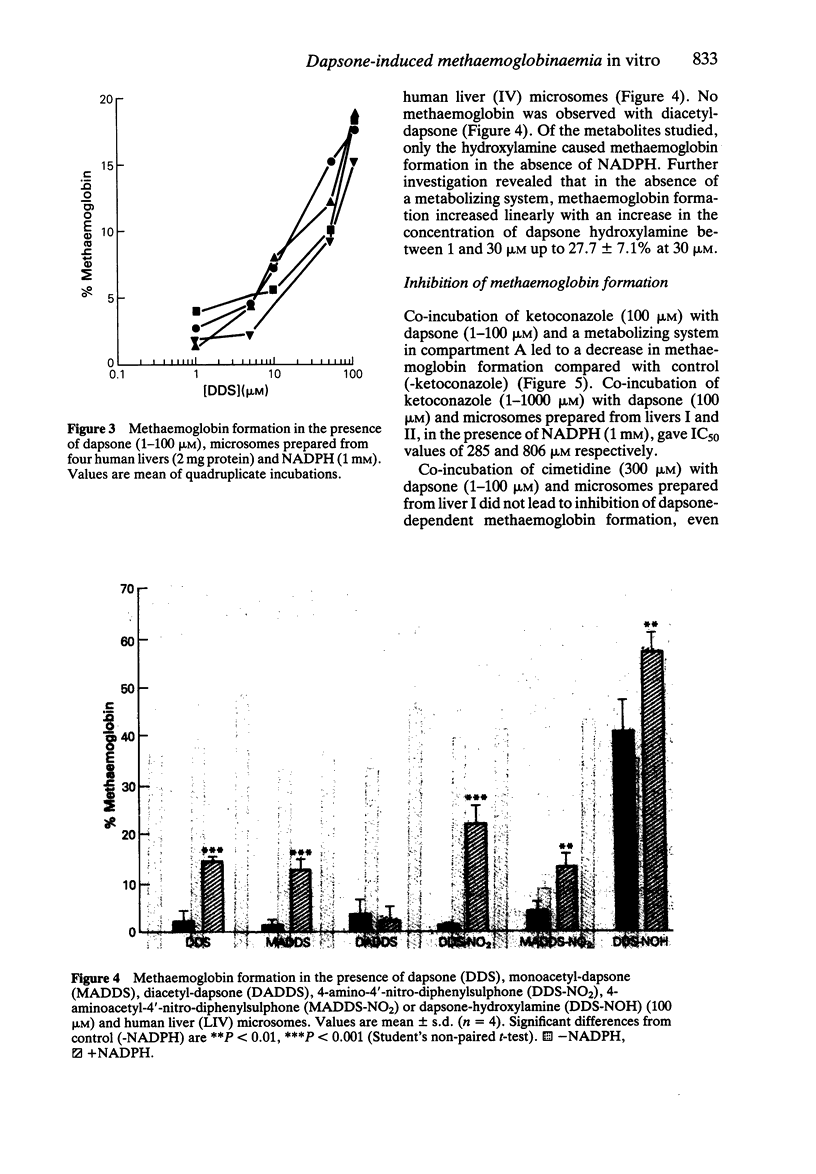
1. We have utilized a two compartment system in which two teflon chambers are separated by a semi-permeable membrane in order to investigate the role of metabolism in dapsone-induced methaemoglobinaemia. Compartment A contained a drug metabolizing system (microsomes prepared from human liver +/- NADPH), whilst compartment B contained target cells (human red cells). 2. Incubation of dapsone (1-100 microM) with human liver microsomes (2 mg protein) and NADPH (1 mM) in compartment A (final volume 500 microliters) led to a concentration-dependent increase in the methaemoglobinaemia (15.4-18.9% at 100 microM) compared with control (2.3 +/- 0.4%) detected in the red cells within compartment B. In the absence of NADPH dapsone had no effect. 3. Of the putative dapsone metabolites investigated, only dapsone-hydroxylamine caused methaemoglobin formation in the absence of NADPH (40.6 +/- 6.3% with 100 microM). However, methaemoglobin was also detected when monoacetyl-dapsone, 4-amino-4'-nitro-diphenylsulphone and 4-aminoacetyl-4'-nitro-diphenylsulphone were incubated with human liver microsomes in the presence of NADPH. 4 Dapsone-dependent methaemoglobin formation was inhibited by addition of ketoconazole (1-1000 microM) to compartment A, with IC50 values of 285 and 806 microM for the two liver microsomal samples studied. In contrast, methaemoglobin formation was not inhibited by cimetidine or a number of drugs pharmacologically-related to dapsone. The presence of glutathione or ascorbate (500 microM) did not alter the level of methaemoglobin observed.
Full text
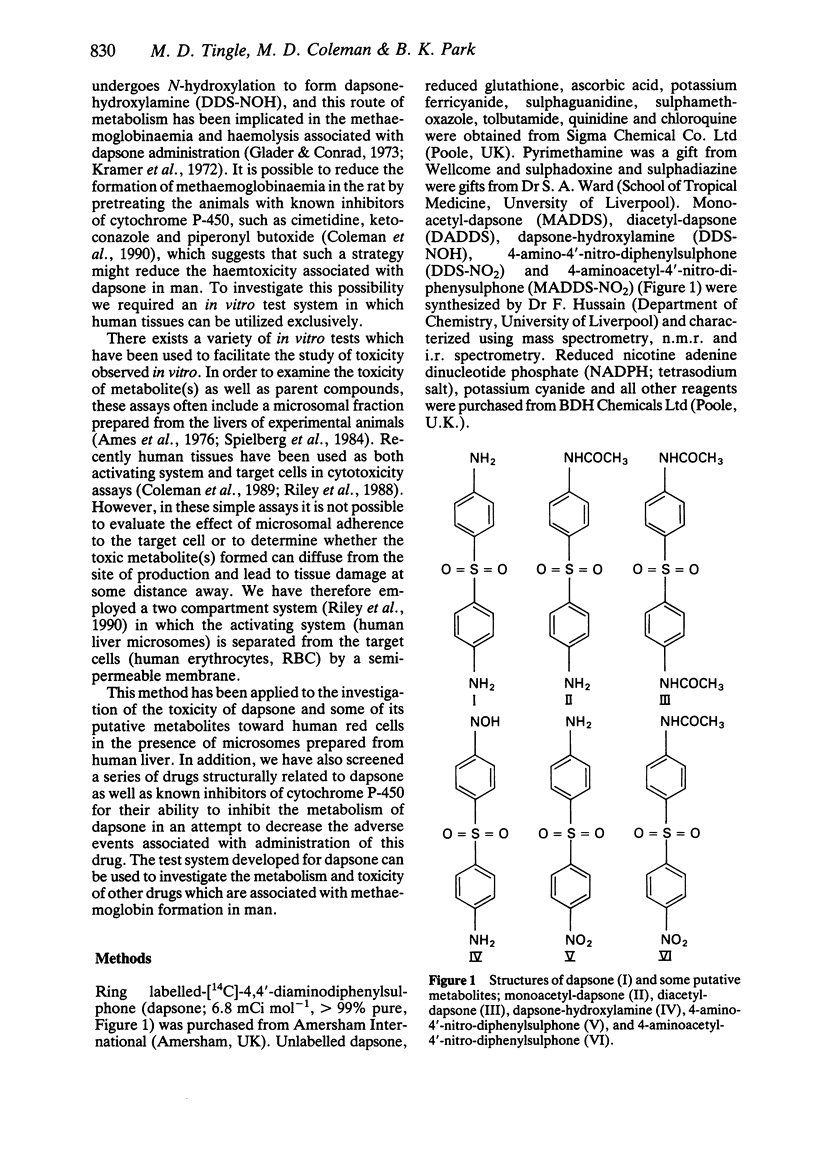
PDF

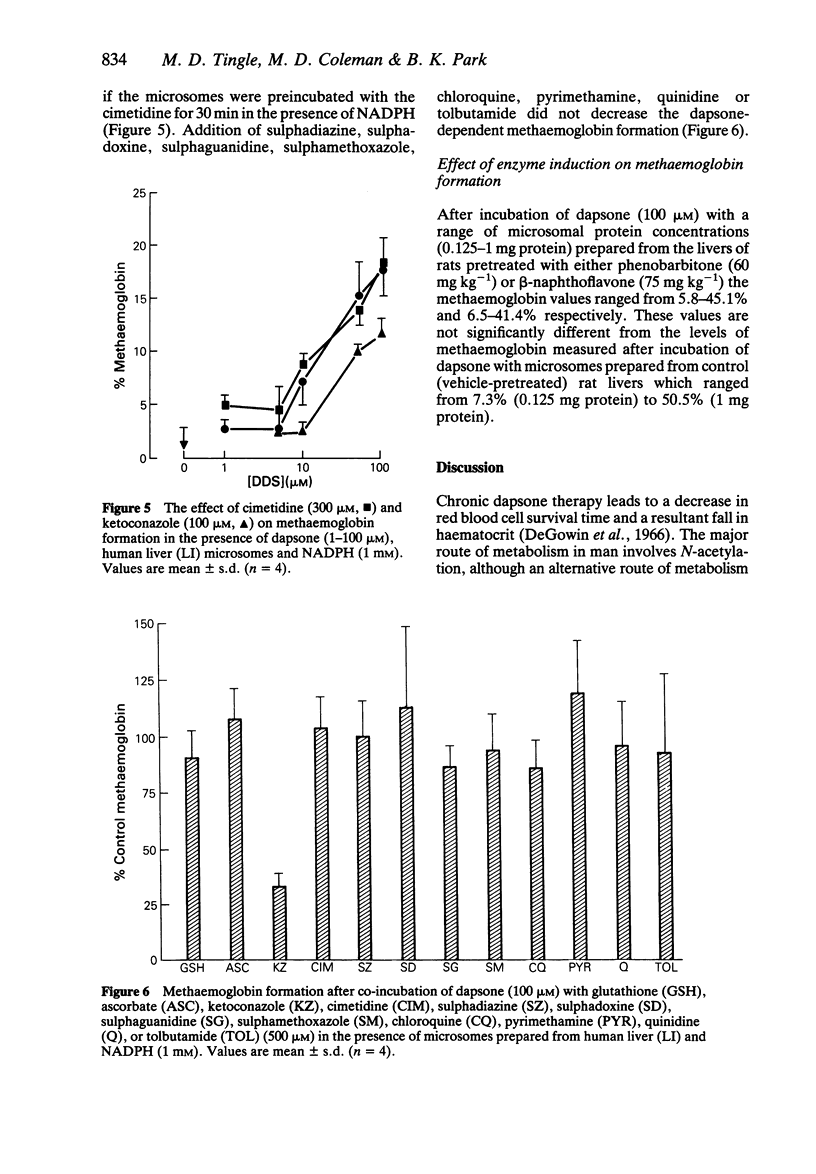



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- BARNES J., BARNES E. J. Liver damage during treatment with diaminodiphenylsulfone. Lepr Rev. 1951 Jul-Oct;22(3-4):54–56. doi: 10.5935/0305-7518.19510008. [DOI] [PubMed] [Google Scholar]
- Coleman M. D., Breckenridge A. M., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite by human hepatic microsomal enzymes. Br J Clin Pharmacol. 1989 Oct;28(4):389–395. doi: 10.1111/j.1365-2125.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. D., Tingle M. D., Winn M. J., Park B. K. Gonadal influence on the metabolism and haematological toxicity of dapsone in the rat. J Pharm Pharmacol. 1990 Oct;42(10):698–703. doi: 10.1111/j.2042-7158.1990.tb06562.x. [DOI] [PubMed] [Google Scholar]
- Coleman M. D., Winn M. J., Breckenridge A. M., Park B. K. Inhibition of dapsone-induced methaemoglobinaemia in the rat. Biochem Pharmacol. 1990 Feb 15;39(4):802–805. doi: 10.1016/0006-2952(90)90164-g. [DOI] [PubMed] [Google Scholar]
- Degowin R. L., Eppes R. B., Powell R. D., Carson P. E. The haemolytic effects of diaphenylsulfone (DDS) in normal subjects and in those with glucose-6-phosphate-dehydrogenase deficiency. Bull World Health Organ. 1966;35(2):165–179. [PMC free article] [PubMed] [Google Scholar]
- Gelber R., Peters J. H., Gordon G. R., Glazko A. J., Levy L. The polymorphic acetylation of dapsone in man. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):225–238. doi: 10.1002/cpt1971122part1225. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Conrad M. E. Hemolysis by diphenylsulfones: comparative effects of DDS and hydroxylamine-DDS. J Lab Clin Med. 1973 Feb;81(2):267–272. [PubMed] [Google Scholar]
- Grindulis K. A., McConkey B. Rheumatoid arthritis: the effects of treatment with dapsone on hemoglobin. J Rheumatol. 1984 Dec;11(6):776–778. [PubMed] [Google Scholar]
- Grossman S. J., Jollow D. J. Role of dapsone hydroxylamine in dapsone-induced hemolytic anemia. J Pharmacol Exp Ther. 1988 Jan;244(1):118–125. [PubMed] [Google Scholar]
- Harrison J. H., Jr, Jollow D. J. Role of aniline metabolites in aniline-induced hemolytic anemia. J Pharmacol Exp Ther. 1986 Sep;238(3):1045–1054. [PubMed] [Google Scholar]
- Israili Z. H., Cucinell S. A., Vaught J., Davis E., Lesser J. M., Dayton P. G. Studies of the metabolism of dapsone in man and experimental animals: formation of N-hydroxy metabolites. J Pharmacol Exp Ther. 1973 Oct;187(1):138–151. [PubMed] [Google Scholar]
- Jensen J. C., Gugler R. Cimetidine interaction with liver microsomes in vitro and in vivo. Involvement of an activated complex with cytochrome P-450. Biochem Pharmacol. 1985 Jun 15;34(12):2141–2146. doi: 10.1016/0006-2952(85)90408-3. [DOI] [PubMed] [Google Scholar]
- Kramer P. A., Glader B. E., Li T. K. Mechanism of methemoglobin formation by diphenylsulfones. Effect of 4-amino-4'-hydroxyaminodiphenylsulfone and other p-substituted derivatives. Biochem Pharmacol. 1972 May 1;21(9):1265–1274. doi: 10.1016/0006-2952(72)90288-2. [DOI] [PubMed] [Google Scholar]
- Lang P. G., Jr Sulfones and sulfonamides in dermatology today. J Am Acad Dermatol. 1979 Dec;1(6):479–492. doi: 10.1016/s0190-9622(79)80088-2. [DOI] [PubMed] [Google Scholar]
- Lenk W., Riedl M. N-hydroxy-N-arylacetamides. V. Differences in the mechanism of haemoglobin oxidation in vitro by N-hydroxy-4-chloroacetanilide and N-hydroxy-4-chloroaniline. Xenobiotica. 1989 Apr;19(4):453–475. doi: 10.3109/00498258909042286. [DOI] [PubMed] [Google Scholar]
- Mitchell M. C., Schenker S., Avant G. R., Speeg K. V., Jr Cimetidine protects against acetaminophen hepatotoxicity in rats. Gastroenterology. 1981 Dec;81(6):1052–1060. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Pelkonen O., Puurunen J. The effect of cimetidine on in vitro and in vivo microsomal drug metabolism in the rat. Biochem Pharmacol. 1980 Nov 15;29(22):3075–3080. doi: 10.1016/0006-2952(80)90448-7. [DOI] [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly P. E., Carrington L. E., Winzor D. J. The interaction of cimetidine with rat liver microsomes. Biochem Pharmacol. 1983 Mar 1;32(5):831–835. doi: 10.1016/0006-2952(83)90584-1. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Maggs J. L., Lambert C., Kitteringham N. R., Park B. K. An in vitro study of the microsomal metabolism and cellular toxicity of phenytoin, sorbinil and mianserin. Br J Clin Pharmacol. 1988 Nov;26(5):577–588. doi: 10.1111/j.1365-2125.1988.tb05298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Roberts P., Coleman M. D., Kitteringham N. R., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite: in vitro use of a novel two compartment system which contains human tissues. Br J Clin Pharmacol. 1990 Sep;30(3):417–426. doi: 10.1111/j.1365-2125.1990.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem Z., Murray T., Yunis A. A. The nitroreduction of chloramphenicol by human liver tissue. J Lab Clin Med. 1981 Jun;97(6):881–886. [PubMed] [Google Scholar]
- Sheets J. J., Mason J. I. Ketoconazole: a potent inhibitor of cytochrome P-450-dependent drug metabolism in rat liver. Drug Metab Dispos. 1984 Sep-Oct;12(5):603–606. [PubMed] [Google Scholar]
- Smith W. C. Are hypersensitivity reactions to dapsone becoming more frequent? Lepr Rev. 1988 Mar;59(1):53–58. doi: 10.5935/0305-7518.19880009. [DOI] [PubMed] [Google Scholar]
- Spielberg S. P. In vitro assessment of pharmacogenetic susceptibility to toxic drug metabolites in humans. Fed Proc. 1984 May 15;43(8):2308–2313. [PubMed] [Google Scholar]
- Uehleke H., Tabarelli S. N-hydroxylation of 4,4'-diaminodiphenylsulphone (Dapsone) by liver microsomes, and in dogs and humans. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(1):55–68. doi: 10.1007/BF00501863. [DOI] [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Shear N. H., Biggar W. D. Metabolism of dapsone to a hydroxylamine by human neutrophils and mononuclear cells. J Pharmacol Exp Ther. 1988 Apr;245(1):274–279. [PubMed] [Google Scholar]
- Wright J. T., Jr, Goodman R. P., Bethel A. M., Lambert C. M. Cimetidine and dapsone acetylation. Drug Metab Dispos. 1984 Nov-Dec;12(6):782–783. [PubMed] [Google Scholar]
- Zuidema J., Hilbers-Modderman E. S., Merkus F. W. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986 Jul-Aug;11(4):299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]


