Abstract
The coding domain of the herpes simplex virus type 1 (HSV-1) α22 gene encodes two proteins, the 420-amino-acid infected-cell protein 22 (ICP22) and US1.5, a protein colinear with the carboxyl-terminal domain of ICP22. In HSV-1-infected cells, ICP22 and US1.5 are extensively modified by the UL13 and US3 viral protein kinases. In this report, we show that in contrast to other viral proteins defined by their properties as α proteins, US1.5 becomes detectable and accumulated only at late times after infection. Moreover, significantly more US1.5 protein accumulated in cells infected with a mutant lacking the UL13 gene than in cells infected with wild-type virus. To define the role of viral protein kinases on the accumulation of US1.5 protein, rabbit skin cells or Vero cells were exposed to recombinant baculoviruses that expressed US1.5, UL13, or US3 proteins under a human cytomegalovirus immediate-early promoter. The results were as follows. (i) Accumulation of the US1.5 protein was reduced by concurrent expression of the UL13 protein kinase and augmented by concurrent expression of the US3 protein kinase. The magnitude of the reduction or increase in the accumulation of the US1.5 protein was cell type dependent. The effect of UL13 kinase appears to be specific inasmuch as it did not affect the accumulation of glycoprotein D in cells doubly infected by recombinant baculoviruses expressing these genes. (ii) The reduction in accumulation of the US1.5 protein was partially due to proteasome-dependent degradation. (iii) Both US1.5 and UL13 proteins activated caspase 3, indicative of programmed cell death. (iv) Concurrent expression of the US3 protein kinase blocked activation of caspase 3. The results are concordant with those published elsewhere (J. Munger and B. Roizman, Proc. Natl. Acad. Sci. USA 98:10410–10415, 2001) that the US3 protein kinase can block apoptosis by degradation or posttranslational modification of BAD.
This report deals with the interaction of three herpes simplex virus (HSV) proteins designated US1.5, UL13, and US3. These proteins are not essential for viral replication for most cells in culture but they appear to play a critical role in experimental animal systems (25, 33–35, 36, 41). Their properties and events that led to these studies were as follows.
The α22 gene contains two discrete transcriptional units, each with its own promoter. The α22 mRNA initiates upstream from the open reading frame (ORF) encoding the 420-amino-acid protein infected-cell protein 22 (ICP22) and is spliced such that the first exon is in the 5′ untranslated region (24, 39, 42). The US1.5 promoter and coding domain are contained entirely within the coding domain of the larger ORF encoding the ICP22 protein (9). The US1.5 ORF is colinear with the middle and carboxyl-terminal domains of the ICP22 ORF. The available evidence indicates that the US1.5 protein is translated from the methionine codon 171 of the ICP22 ORF (A. P. W. Poon, W. O. Ogle, and B. Roizman, unpublished results). Both ORFs are transcribed in cells infected and maintained in the presence of cycloheximide, and both proteins are made upon withdrawal of the drug. On that basis, they have been classified as α proteins (9, 16, 17). ICP22 is extensively posttranslationally modified by two viral protein kinases encoded by the US3 and UL13 genes and by unidentified cellular kinases, and ICP22 is also nucleotidylated by casein kinase II (1, 23, 26, 27, 31, 35, 36). UL13 has also been shown to modify the US1.5 protein (31). Both an intact α22 gene and the UL13 protein kinase are required for the expression of a subset of γ2 viral genes expressed late in infection exemplified by the products of the US11 and UL38 ORFs (31, 35). The accumulation of US11 and UL38 proteins in cells infected with a mutant virus expressing the US1.5 protein but not ICP22 is similar to that observed in wild-type virus infection, and posttranslational modification of US1.5 by UL13 is required for this accumulation of γ2 gene products (31). Additional evidence supporting the conclusion that US1.5 mediates the accumulation of this subset of γ2 gene products is that posttranslational processing of ICP22 is not essential for accumulation of US11 or UL38 proteins (32).
However, the mutant virus which expresses US1.5 protein but not ICP22 is highly attenuated in mice, suggesting that the sequences unique to ICP22 perform functions other than those of sequences shared by ICP22 and US1.5 (31). Furthermore, the insertion of a 20-codon linker at codon 200 or 240 of ICP22 had no apparent effect on functions associated with ICP22 and US1.5 (9).
As evidence has emerged that the function of these proteins is determined by the extent of posttranslational modification (31), one objective of the studies on these proteins was to map the sites of phosphorylation by both cellular and viral kinases. The phenotype of a mutant virus with a defective UL13 gene is very similar to that of a virus with a defective α22 gene (35), suggesting that modification of ICP22 and/or US1.5 is an important function of UL13 in the viral life cycle. Initial attempts to map these proteins by mutagenesis led to the discovery that amino acid substitution near the amino terminus of ICP22 can preclude the phosphorylation of ICP22 at sites near its carboxyl terminus (32). One alternative was to produce the proteins in isolation in mammalian cells and identify the sites by other means.
The UL13 protein kinase targets a large number of both viral and cellular substrates (reviewed in reference 40). As noted above, UL13 protein kinase appears to regulate the function of viral proteins. The protein kinase mediates the hyperphosphorylation of the translation initiation factor 1δ and the activation of cdc25C phosphatase (2, 20).
Until recently, little was known about US3 protein kinase other than a limited number of proteins whose posttranslational modification appears to be mediated by this enzyme (reviewed in reference 40). The connection to apoptosis was based on studies of the d120 mutant, in which both copies of the α4 gene encoding ICP4, the major regulatory protein of the virus, had been deleted (11). This mutant induced apoptosis in all cell lines tested (14, 15, 21). A recombinant virus derived by restoration of the α4 genes also induced programmed cell death, and only coexpression of a fragment encoding the US3 gene blocked apoptosis (22, 28). The apparent role of US3 in blocking apoptosis was confirmed in other studies. Whereas a wild-type virus blocked apoptosis induced by UV light irradiation or anti-fatty acid synthase (FAS) antibody, a mutant lacking the US3 gene failed to do so (19). In vivo, the US3 gene is required for the protection of corneal epithelial cells and primary afferent neurons from apoptosis in infected mice (3, 4). The mechanism by which US3 blocks apoptosis emerged in two recent studies. In the first, US3 provided in trans blocked apoptosis induced by the d120 mutant described above at a premitochondrial stage consistent with evidence that apoptosis was also blocked by overexpression of Bcl-2 (15, 28). The second and more recent study showed that the US3 protein kinase blocks apoptosis induced by BAD, a proapoptotic member of the Bcl-2 family of proteins (29).
To express the US1.5 protein in mammalian cells with high efficiency, we cloned the ORF in baculoviruses under the human cytomegalovirus immediate-early promoter. The objective of the studies was to isolate the unmodified forms as well as the forms posttranslationally modified by either the US3 or UL13 protein kinases whose genes were also cloned in baculoviruses.
We report that whereas US3 augmented the amounts of US1.5 that accumulated in Vero cells exposed to recombinant baculoviruses expressing both US3 and US1.5, the accumulation of US1.5 protein was significantly reduced in cells doubly infected with baculoviruses expressing US1.5 and UL13. The results indicate that cells infected with US1.5 or UL13 contain activated caspase 3 characteristic of apoptosis and that the US3 protein kinase effectively blocks the activation of caspase 3, as would be predicted from its effectiveness in blocking apoptosis in infected cells.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were obtained from the American Type Culture Collection (Manassas, Va.), and rabbit skin cells were originally obtained from J. McClaren. Cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (rabbit skin cells) or 5% newborn calf serum (Vero cells). Insect cell line Sf9 (Spodoptera frugiperda) was obtained from PharMingen (San Diego, Calif.). HSV-1(F) is the prototype HSV-1 strain used in this laboratory (13). The construction of HSV-1 recombinant viruses R7041 (ΔUS3), R7353 (ΔUL13/ΔUS3), and R7356 (ΔUL13) was previously described (34–36). Wild-type baculovirus was obtained from PharMingen. The construction of recombinant baculoviruses Bac-glycoprotein D (Bac-gD) and Bac-US3 was described elsewhere (28, 29, 44).
Plasmids.
pRB5252 contains the entire ICP22 ORF in vector pUC19 and thus contains US1.5 (32). The DNA fragment containing the putative US1.5 ORF (amino acids 171 to 420) was generated by PCR using pRB5252 as the template and primers AP1 (GGG AAT TCA TGC TAC GGC GCT CGG TG) and AP2 (ACG CGT CGA CCT ACG GCC GGA GAA A). AP1 included in the final product restriction site EcoRI (underlined) at the 5′ end of the US1.5 ORF, and AP2 incorporated a SalI site (underlined) at the 3′ end. The EcoRI/SalI-digested PCR product was purified and subcloned into the EcoRI-SalI site of vector pGex 4T-1 (Amersham Pharmacia Biotech, Piscataway, N.J.). The resulting plasmid was designated pRB5450.
Baculovirus transfer vectors.
pRB5450 was digested with EcoRI and NotI, and the resulting fragment containing the US1.5 ORF was purified and subcloned into the EcoRI-NotI site of the previously described baculovirus transfer vector pAc-CMV (44). The resulting plasmid was designated pAc-US1.5. pRB5151 contains the entire UL13 coding sequence in the pGex 4T-1 vector (30). pRB5151 was digested with EcoRI and NotI, and the resulting fragment containing the UL13 ORF was purified and subcloned into the EcoRI-NotI site of pAc-CMV. The resulting plasmid was designated pAc-UL13.
Generation of recombinant baculovirus.
Bac-US1.5 and Bac-UL13 were generated using the PharMingen baculovirus expression vector system by cotransfecting transfer vectors pAC-US1.5 and pAC-UL13, respectively, along with BaculoGold linearized baculovirus DNA (PharMingen) into Sf9 cells according to the manufacturer’s instructions. Viruses were propagated in Sf9 cells grown in 20 ml of TNM-FH insect cell medium (PharMingen) in 150-cm2 flasks. The supernatant containing virus was harvested and cleared by centrifugation at 1,000 rpm for 5 min at 4°C.
Preparation of cell lysates, electrophoretic separation, and immunoblotting of viral proteins from HSV-1-infected cells.
Replicate cultures of Vero cells or rabbit skin cells in 25-cm2 flasks were either mock infected or exposed to 5 PFU of virus per cell and maintained at 37°C in medium 199V (medium 199 supplemented with 1% calf serum). Cells were harvested at the indicated time after infection, washed three times with phosphate-buffered saline (PBS), and then solubilized in 200 μl of disruption buffer (50 mM Tris-HCl [pH 7], 2% sodium dodecyl sulfate [SDS], 710 mM β-mercaptoethanol, 3% sucrose). After 50-μl aliquots of lysate were boiled for 5 min, solubilized proteins were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 11% bisacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, reacted with primary antibody followed by appropriate secondary antibody conjugated to alkaline phosphatase (Bio-Rad Laboratories, Hercules, Calif.), and visualized according to the manufacturer’s instructions with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium) (Sigma).
Exposure of mammalian cells to recombinant baculovirus.
Subconfluent cultures of rabbit skin in 25-cm2 flasks were incubated with baculovirus in a total volume of 1 ml for 1 h at 37°C. Then, 500 μl of baculovirus stock was used for each virus per infection unless otherwise indicated. For single infections, the volume was brought up to 1 ml with 199V. Culture medium was then replaced with fresh DMEM containing 5% newborn calf serum and 10 mM sodium butyrate unless otherwise indicated and was incubated at 37°C for 24 h. For experiments involving MG132 treatment, culture medium was replaced with fresh DMEM containing 5% newborn calf serum, 10 mM sodium butyrate, and 5 μM MG132 (BioMol, Plymouth Meeting, Pa.) at the indicated time before harvesting at 24 h after infection.
One-dimensional electrophoretic analysis of recombinant HSV-1 proteins.
Cells infected with recombinant baculovirus were scraped, washed twice with PBS, and lysed in PBS-A* (1% Nonidet P-40 and 1% deoxycholate in PBS) containing 0.1 mM TLCK (Nα-p-tosyl-ℒ-lysine chloromethyl ketone), 0.1 mM TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone), 1 mM phenylmethylsulfonyl fluoride, Pefabloc SC (1 mg/ml; Boehringer Mannheim, Mannheim, Germany), EDTA (0.5 mg/ml), leupeptin (10 μg/ml), pepstatin (10 μg/ml), aprotinin (1 μg/ml), and E-64 (10 μg/ml; Boehringer Mannheim) or PBS-A* in which complete, Mini, EDTA-free protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany) had been dissolved according to the manufacturer’s instructions. The amount of total protein in the lysate was quantified using the Bio-Rad Protein Assay according to the manufacturer’s instructions. Solubilized proteins were subjected to SDS-PAGE on 12% bisacrylamide gels. Immunoblotting was performed as described above.
Two-dimensional electrophoretic analysis of recombinant HSV-1 proteins.
Two-dimensional electrophoresis was performed using an immobilized pH gradient (IPG) for first-dimension isoelectric focusing (8). A protocol previously described (7) was modified. Briefly, rabbit skin cells infected with recombinant baculovirus were washed twice with PBS and lysed in 80 μl of lysis solution {8 M urea, 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 40 mM Tris base} containing 0.1 mM TLCK, 0.1 mM TPCK, 1 mM phenylmethylsulfonyl fluoride, Pefabloc SC (1 mg/ml; Boehringer Mannheim), EDTA (0.5 mg/ml), leupeptin (10 μg/ml), pepstatin (10 μg/ml), aprotinin (1 μg/ml), and E-64 (10 μg/ml, Boehringer Mannheim). The extract was kept on ice for 1 h and then sonicated, and the insoluble material was pelleted by centrifugation. The soluble fraction was transferred to a new tube, and 170 μl of rehydration stock solution (8 M urea, 2% CHAPS, 20 mM dithiothreitol [DTT], bromophenol blue) was added to the sample for a total volume of 250 μl. pH 3 to 10 L IPG Buffer (Amersham Pharmacia Biotech catalog no. 17-6000-87) was added for a final concentration of 0.5%. First-dimension isoelectric focusing (IEF) on 13-cm Immobiline DryStrip gels (linear pH 3 to 10 gradient) (Amersham Pharmacia Biotech catalog no. 17-6001-14) was performed using an IPGphor Isoelectric Focusing System (Amersham Pharmacia Biotech). IPG strips were rehydrated in the sample solution for 12 h and subjected to the following procedures: 500 V for 1 h, 1,000 V for 1 h, and 8,000 V for 2 h for a total of 17,500 V h at 20°C. IEF gels were then equilibrated for 15 min in SDS equilibration buffer (50 mM Tris-HCl [pH 8.8], 6 M urea, 30% glycerol, 2% SDS, 10 mg of DTT/ml, bromophenol blue). Equilibrated strips were overlaid onto 12% bisacrylamide gels and sealed with agarose sealing solution (0.5% agarose, 25 mM Tris base, 192 mM glycine, 0.1% SDS, bromophenol blue). Molecular weight markers were placed adjacent to the pH 10 end of the IPG strip on an IEF electrode strip (Amersham Pharmacia Biotech catalog no. 18-1004-40). Second-dimension SDS-PAGE gels were subjected to 20 mA for the first hour, followed by 30 mA. Immunoblotting of electrophoretically separated proteins was performed as described above.
Antibodies.
Rabbit polyclonal antibody W2 made against the carboxyl terminus of ICP22/US1.5 was described elsewhere (23) and used at a 1:3,000 dilution for Western blotting. Mouse monoclonal antibody against gD (clone H170) purchased from the Rumbaugh-Goodwin Cancer Research Institute (Plantation, Fla.) was described elsewhere (44) and was used at a 1:2,500 dilution for Western blotting.
Measurement of DEVDase activity.
Caspase 3 activity in cellular extracts was assayed using a tetrapeptide conjugated to phenylnitraniline (DEVD-pNA) (BioMol). Rabbit skin cells grown in 25-cm2 flasks were either mock infected or infected with the indicated recombinant baculovirus or combination of baculoviruses as described above. At 24 h after infection, the cells were scraped in their medium, washed twice with PBS, resuspended in 150 μl of lysis solution (0.1% CHAPS, 50 mM HEPES [pH 7.4], 1 mM DTT, 0.1 mM EDTA), and incubated on ice for 5 min. Lysates were then centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was collected and analyzed for DEVDase activity according to the manufacturer’s instructions. Chromophore release was quantified by measuring absorbance at 405 nm with a spectrophotometer after 2 h.
RESULTS
Kinetics of accumulation of US1.5 protein.
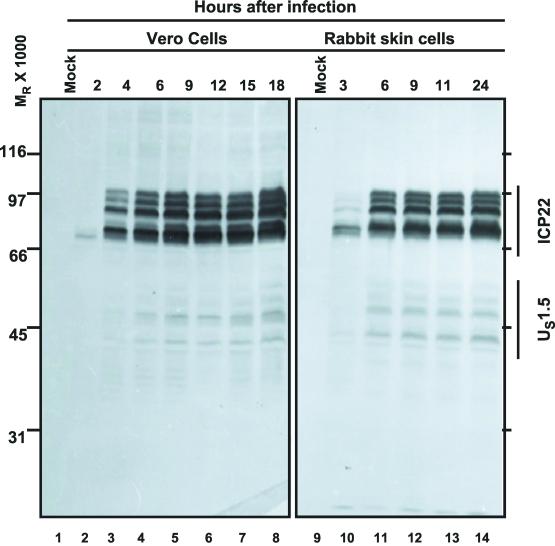
Replicate cultures of Vero or rabbit skin cells were exposed to 5 PFU of HSV-1(F) per cell. In the experiment shown in Fig. 1, the cells were harvested at 2, 4, 6, 9, 12, 15, or 18 h after infection whereas the rabbit skin cells were harvested at 3, 6, 9, 11, or 24 h after infection. The harvested cells were solubilized, and the proteins were subjected to electrophoresis in denaturing gels and then reacted with rabbit polyclonal antibody directed to ICP22/US1.5 protein as described in Materials and Methods. The results were as follows: ICP22 appeared as a faint band in lysates of Vero cells harvested at 2 h after infection (Fig. 1, lane 2). Fully processed forms of ICP22 were detected in lysates of cells harvested at 4 h after infection (Fig. 1, lane 3). From 6 to 18 h after infection, the amounts of ICP22 present in cell lysates increased, but very gradually, suggesting that the peak rates of synthesis were between 2 and 6 h after infection (Fig. 1). In contrast, US1.5 was barely detectable at 2 h after infection and continued to increase in amount until 18 h after infection (Fig. 1, lane 8). In other experiments (data not shown), we found that US1.5 continued to increase for as long as 30 h after infection. In rabbit skin cells, both ICP22 and US1.5 protein was detected at 3 h after infection. In these cells, there was no significant increase in accumulation of either protein past 6 h after infection (Fig. 1, lanes 11 to 14).
FIG. 1.
Accumulation of ICP22/US1.5 proteins in HSV-1(F)-infected cells. Replicate cultures of Vero cells or rabbit skin cells were exposed to 5 PFU of HSV-1(F) per cell. Cells were harvested at the indicated times after infection, solubilized, subjected to SDS-PAGE, transferred to nitrocellulose sheets, and reacted with rabbit polyclonal antibody directed to the carboxyl-terminal domain of ICP22/US1.5 proteins as described in Materials and Methods.
Viral protein kinases negatively regulate US1.5 levels in infected cells.
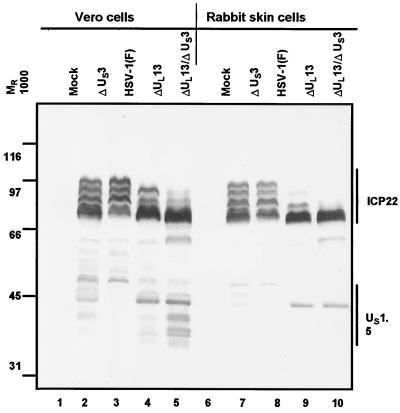
In this series of experiments, replicate cultures of Vero or rabbit skin cells were exposed to 5 PFU of wild-type HSV-1(F) or mutants lacking UL13 (R7356), US3 (R7041), or both UL13 and US3 ORFs (R7353) per cell. The cells were harvested at 18 h after infection and processed as described in Materials and Methods. The results were as follows. (i) As expected, both UL13 and US3 protein kinases posttranslationally modify ICP22 (Fig. 2 and references 35 and 36). Furthermore, as expected on the basis of earlier reports from this laboratory, the presence and accumulation of slow-migrating forms of ICP22 were affected by the absence of one or both protein kinases (Fig. 2). As the absence of the UL13 kinase has a more pronounced effect ablating slow-migrating forms than the absence of US3 alone and appears to be responsible for the loss of most of the forms when both kinases are absent (Fig. 2, compare lanes 3, 4, and 5 and lanes 8, 9, and 10), these data confirm previous results showing that the UL13 protein kinase mediates more extensive modification of ICP22 in infected cells than the US3 protein kinase (Fig. 2 and references 35 and 36).
FIG. 2.
Accumulation of ICP22/US1.5 proteins in cells infected with wild-type and mutant viruses. Replicate cultures of Vero cells or rabbit skin cells were exposed to 5 PFU of HSV-1(F) or mutants lacking US3 (R7041), or UL13 (R7356) or both UL13 and US3 (R7353) genes per cell. Cells were harvested at 18 h after infection and processed as described in the legend to Fig. 1.
(ii) As described elsewhere, the UL13 protein kinase also posttranslationally modifies US1.5 (31). In Vero cells infected with HSV-1(F), the US1.5 protein was detected as a faint, slow-migrating band with an apparent Mr of approximately 50,000 (Fig. 2, lane 3). The US1.5 protein was most abundant and formed numerous faster-migrating bands in cells infected with ΔUL13/ΔUS3 mutant virus (Fig. 2, lane 5), and it was intermediate in abundance and also in electrophoretic mobility in lysates of cells infected with the ΔUS3 mutant virus (Fig. 2, lane 2). Cells infected with the ΔUL13 mutant formed a somewhat abundant band with an Mr of approximately 43,000 and a small number of faint bands migrating more slowly (Fig. 2, lane 4). In Vero cells, rapidly migrating bands observed in lysates of cells infected with the ΔUL13 virus which are not present in cells infected with wild-type virus (Fig. 2, compare lanes 3 and 4) reflect the lack of modification of US1.5 by UL13. Such rapidly migrating bands are present to a lesser extent and do not migrate as rapidly in ΔUS3 virus infection (Fig. 2, lane 2) and are more prominent in lysates of cells infected with the ΔUL13/ΔUS3 mutant virus than with the single ΔUL13 kinase deletion mutant (Fig. 2, compare lanes 4 and 5). These data suggest that the US3 protein kinase mediates posttranslational modification of the US1.5 protein, albeit to a lesser extent than the UL13 protein kinase.
(iii) The US1.5 protein was barely detectable in rabbit skin cells infected with the wild-type virus or ΔUS3 mutant. In cells infected with the ΔUL13 or ΔUL13/ΔUS3 mutant, US1.5 formed a single band with an apparent Mr of 43,000 (Fig. 2, lanes 9 and 10).
We conclude from these studies that the accumulation of US1.5 protein is regulated directly or indirectly by viral protein kinases. The amounts of US1.5 were lowest in lysates of cells infected with wild-type virus. The effect of the kinases appeared to be cell type dependent. In infected Vero cells, both US3 and UL13 kinases mediated an inhibitory effect on US1.5 accumulation which was additive, whereas in rabbit skin cells, the repressive effect appeared to be mediated primarily by the UL13 protein kinase.
US1.5 protein is expressed in cells infected with recombinant baculovirus carrying US1.5 ORF.
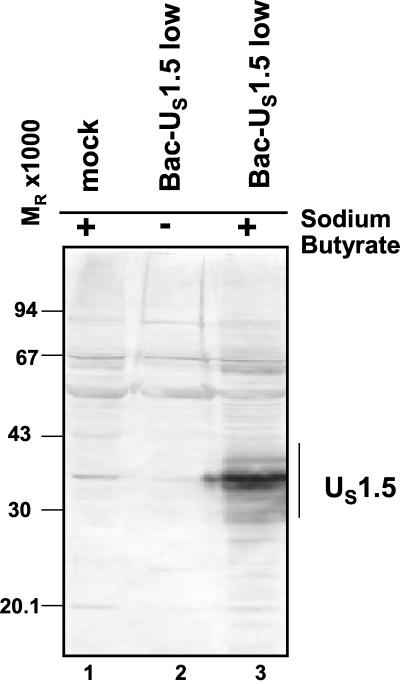
Both US1.5 and ICP22 have been shown to be phosphorylated by the HSV protein kinases. To investigate more closely the mechanisms by which viral protein kinases affect the accumulation of the US1.5 protein, it was necessary to express US1.5 and the protein kinases independently of other viral proteins. To this end, the sequence encoding the US1.5 ORF driven by the cytomegalovirus (CMV) immediate-early promoter was cloned into a baculovirus. In the experiments illustrated in Fig. 3, rabbit skin cells were exposed to approximately 10 PFU of baculovirus per cell and incubated in the presence or absence of sodium butyrate. Efficient expression of baculovirus genes in mammalian cells has previously been shown to require treatment with sodium butyrate, a histone deacetylase inhibitor (10). The cells were harvested 24 h after infection and solubilized, and the proteins were electrophoretically separated in a denaturing gel and probed with antibody to ICP22/US1.5 protein. The results shown in Fig. 3 indicate that the US1.5 protein readily accumulated in rabbit skin cells infected with the recombinant baculovirus and maintained in the presence of sodium butyrate. We should note, however, that the US1.5 expressed by baculoviruses in Vero cells or rabbit skin cells migrated significantly faster than that expressed in cells infected with wild-type or mutant viruses (compare Fig. 1 and 2 with Fig. 3). The results suggest that US1.5 protein made in HSV-1-infected cells undergoes posttranslational modifications other than those mediated by the two viral protein kinases and which were not observed in cells exposed to the recombinant baculoviruses expressing the US1.5 ORF. These modifications are likely due to modulation of the activity of cellular enzymes by HSV-1 infection which does not occur when US1.5 is transduced in the absence of other viral proteins.
FIG. 3.
Accumulation of US1.5 protein in cells infected with recombinant baculovirus containing US1.5 gene. Replicate cultures of rabbit skin cells were either mock infected or exposed to approximately 5 PFU of Bac-US1.5 per cell and cultured in the presence or absence of 10 mM sodium butyrate as indicated. Cells were harvested 24 h after baculovirus infection, solubilized, subjected to electrophoresis in denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with rabbit polyclonal antibody directed against the carboxyl-terminal domain of ICP22/US1.5 proteins as described in Materials and Methods.
Accumulation of US1.5 protein is differentially modulated by viral protein kinases in cells exposed to recombinant baculoviruses expressing US1.5 and each of the viral protein kinases.
The purpose of this series of experiments was to determine the effects of protein kinases on the accumulation of US1.5 protein in the absence of other viral proteins. To this end, replicate cultures of Vero cells or rabbit skin cells were exposed to approximately 10 PFU of baculovirus encoding US1.5 (Bac-US1.5) per cell alone or in combination with baculoviruses encoding US3 (Bac-US3) or UL13 (Bac-UL13) genes (10 PFU of each per cell) in the presence or absence of the proteasomal inhibitor MG132. Two series of experiments were done.
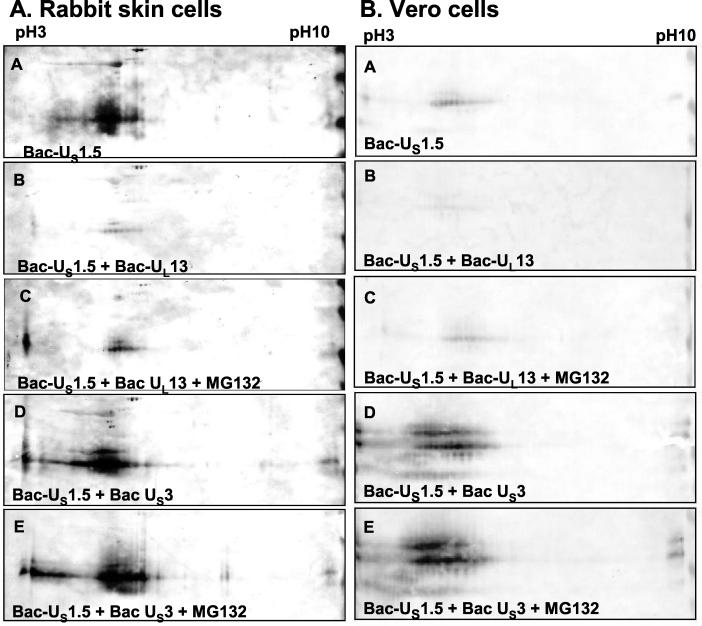
In the first series, cells were harvested at 24 h after infection and solubilized, and lysate containing 100 μg of total protein from each sample was subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to nitrocellulose sheets, and reacted with antibody to the ICP22/US1.5 protein (Fig. 4). In the second series of experiments, the baculovirus-infected cell lysates were subjected to two-dimensional separation and reacted with the same antibody. The results shown in Fig. 5A (rabbit skin cells) and Fig. 5B (Vero cells) were as follows.
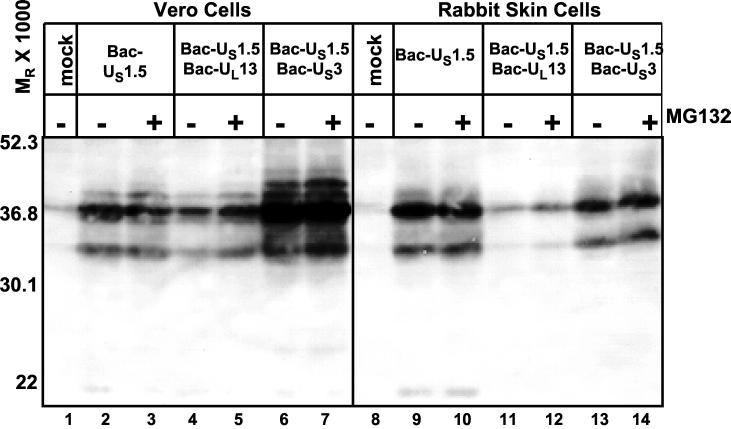
FIG. 4.
Effect of viral kinase expression on accumulation of US1.5 protein expressed via recombinant baculovirus in Vero cells or rabbit skin cells. Replicate cultures were either mock infected or infected with approximately 10 PFU of the indicated baculovirus or baculoviruses and cultured in the presence of 10 mM sodium butyrate. Cells were harvested 24 h after baculovirus infection. Six hours prior to harvesting, the cultures were replenished with fresh medium containing 5 μM MG132 and 10 mM sodium butyrate where indicated. Cells were then solubilized, and lysates containing 100 μg of total protein were subjected to electrophoresis in denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with rabbit polyclonal antibody directed against the carboxyl-terminal domain of ICP22/US1.5 proteins.
FIG. 5.
Effect of viral protein kinase expression on posttranslational modification and accumulation of US1.5 protein expressed via recombinant baculovirus in rabbit skin cells (A) or Vero cells (B). Replicate cultures were either mock infected or infected with approximately 10 PFU of the indicated baculovirus or baculoviruses and maintained in the presence of 10 mM sodium butyrate. Cells were harvested 24 h after baculovirus infection. Six hours prior to harvesting, the cultures were replenished with fresh medium containing 5 μM MG132 and 10 mM sodium butyrate. Cells were solubilized in two-dimensional gel lysis solution, and two-dimensional electrophoretic separations were done as described in Materials and Methods. Electrophoretically separated proteins were transferred to nitrocellulose sheets and reacted with rabbit polyclonal antibody directed against the carboxyl-terminal domain of ICP22/US1.5 proteins.
(i) Two-dimensional electrophoretic separations of lysates of rabbit skin cells and Vero cells infected with Bac-US1.5 show that US1.5 protein formed multiple isoforms with different isoelectric points (pI) (Fig. 5A and B, panel A). This observation indicates that US1.5 is posttranslationally modified (likely by phosphorylation) by cellular enzymes in the absence of any other viral protein expression.
(ii) The amounts of US1.5 protein expressed in Vero cells or in rabbit skin cells doubly infected with Bac-US1.5 and Bac-UL13 were significantly smaller than those in cells infected with Bac-US1.5 only. This effect of UL13 transduction on US1.5 protein expression was more pronounced in rabbit skin cells than in Vero cells and was observed in both one-dimensional separations (Fig. 4, compare lanes 2 and 4 and lanes 9 and 11) and two-dimensional separations (Fig. 5A and B, compare panels A and B).
(iii) A central question arising from the results described above was whether the UL13 protein kinase mediates the proteasome-dependent degradation of the US1.5 protein. As shown in Fig. 4, the incubation of infected rabbit skin cells and Vero cells in medium containing the proteasome inhibitor MG132 for 6 h prior to harvesting increased the amounts of US1.5 protein in cells doubly infected with Bac-US1.5 and Bac-UL13 compared to cells cultured in medium without MG132 (compare lanes 4 and 5 and lanes 11 and 12). The effect of MG132 was more pronounced in Vero cells than in rabbit skin cells but was apparent in rabbit skin cells, especially with regard to the low-molecular-weight isoform. The increase in the amounts of US1.5 protein in rabbit skin cells and Vero cells doubly infected with Bac-US1.5 and Bac-UL13 was also apparent on two-dimensional separations of the cell lysates (Fig. 5A and B, compare panels B and C). This result indicates that UL13 expression effects proteasome-dependent degradation of the US1.5 protein. If UL13 targets US1.5 for proteasome-dependent degradation, US1.5 protein would have accumulated only in cells transduced with US1.5 and UL13 for the period of MG132 treatment while the protein would have accumulated for the entire duration of baculovirus infection when cells were transduced with US1.5 alone. Therefore, the observation that MG132 treatment of cells transduced with US1.5 and UL13 resulted in less US1.5 protein accumulation than transduction of US1.5 alone (Fig. 4, compare lanes 1 and 5 and lanes 9 and 12; Fig. 5A and B, compare panels A and C) is consistent with and supports this conclusion.
(iv) Treatment of rabbit skin cells coinfected with Bac-US1.5 and Bac-UL13 with MG132 for 2 h prior to harvesting increased the accumulation of US1.5 compared to untreated coinfected cells, but less US1.5 was present compared to coinfected cells treated with MG132 for 6 h before harvesting (data not shown). This result suggests that US1.5 protein is constitutively synthesized and degraded in a proteasome-dependent manner in cells coinfected with Bac-US1.5 and Bac-UL13 and accumulates when the proteasome-dependent degradation machinery is inactivated.
(v) Low levels of a highly electronegative form of US1.5 were present in rabbit skin cells coinfected with Bac-US1.5 and Bac-UL13, a form that was not present in cells infected with Bac-US1.5 alone (Fig. 5A, compare panels A and B). This highly electronegative US1.5 isoform was very prominent in lysates of rabbit skin cells coinfected with Bac-US1.5 and Bac-UL13 which were exposed to MG132 (Fig. 5A, panel C). This isoform was also faintly visible in two-dimensional separations of lysates of Vero cells coinfected with Bac-US1.5 and Bac-UL13 and treated with MG132 but was not detectable in untreated coinfected cells (Fig. 5B, panels B and C). The appearance of a highly electronegative US1.5 isoform in lysates of treated cells when the UL13 protein kinase is also transduced suggests more extensive phosphorylation of US1.5. It is of note that the observed effect of MG132 was more pronounced in two-dimensional separations than in one-dimensional separations because the electronegative isoform is not resolved by conventional one-dimensional SDS-PAGE. Accumulation of electronegative isoforms specifically when the proteasome is inactive indicates that the UL13 protein kinase posttranslationally modifies the US1.5 protein and US1.5 isoforms modified by UL13 are extensively degraded in a proteasome-dependent manner.
(vi) In rabbit skin cells coinfected with Bac-US1.5 and Bac-gD, a recombinant baculovirus expressing the HSV-1 glycoprotein D (gD), US1.5 accumulated to the same level as in cells infected with Bac-US1.5 alone (data not shown), suggesting that the negative effect of coinfection of Bac-UL13 with Bac-US1.5 on US1.5 expression is specifically mediated by the UL13 gene and is not due to competition with Bac-US1.5 for cellular receptors during viral entry or for transcription factors which drive expression from the CMV promoter.
(vii) Treatment with MG132 did not affect US1.5 accumulation in rabbit skin cells and Vero cells infected with Bac-US1.5 (Fig. 4, compare lanes 2 and 3 and lanes 9 and 10), suggesting that US1.5 is not constitutively degraded in a proteasome-dependent manner to a significant extent in the absence of UL13 expression.
(viii) The amounts of US1.5 protein expressed in Vero cells doubly infected with Bac-US1.5 and Bac-US3 were vastly greater than those in cells infected with Bac-US1.5 only. This was observed in both one-dimensional separations (Fig. 4, compare lanes 2 and 6) and two-dimensional separations (Fig. 5B, compare panels A and D). It is noteworthy that in the lysates of cells doubly infected with Bac-US1.5 and Bac-US3, US1.5 protein formed multiple bands of differential electrophoretic mobilities indicative of posttranslational processing. In rabbit skin cells, the total amounts of US1.5 protein present in lysates of cells singly infected with Bac-US1.5 or both Bac-US1.5 and Bac-US3 were approximately the same (Fig. 4, compare lanes 9 and 13, and Fig. 5A, compare panels A and D). A notable difference is that in rabbit skin cells, the US1.5 protein formed multiple bands differing in electrophoretic mobility on two-dimensional separations, suggesting that it was posttranslationally modified in both the absence and presence of US3 protein kinase (Fig. 5A, panels A and D). However, highly electronegative forms of US1.5 which were not present in cells infected with Bac-US1.5 alone were observed in rabbit skin cells and Vero cells coinfected with Bac-US1.5 and Bac-US3 (Fig. 5A and B, compare panels A and D). The presence of these electronegative forms is indicative of a more extensive modification of US1.5, which is likely phosphorylation in cells coinfected with Bac-US1.5 and Bac-US3 compared to cells infected with Bac-US1.5 alone. Thus, these data suggest that US3 protein kinase mediates the posttranslational modification of transduced US1.5 protein in both rabbit skin cells and Vero cells.
(ix) MG132 treatment had no effect on the levels or posttranslational modification of US1.5 in rabbit skin cells and Vero cells coinfected with Bac-US1.5 and Bac-US3 (Fig. 4, compare lanes 6 and 7 and lanes 13 and 14; Fig. 5A and B, compare panels D and E). Thus, unlike that of UL13, US3 function does not mediate proteasome-dependent degradation of the US1.5 protein.
(x) Treatment of rabbit skin cells and Vero cells infected with HSV-1(F) with MG132 during the course of infection caused increased accumulation of US1.5 compared to untreated infected cells (data not shown).
Therefore, these results indicate that both HSV-1 viral kinases, UL13, and US3 mediate posttranslational modification of the US1.5 protein but with differential effects. Thus, modification of US1.5 by UL13 targets US1.5 for proteasome-dependent degradation whereas US3 expression leads to extensive modification of US1.5 but does not cause the degradation of US1.5.
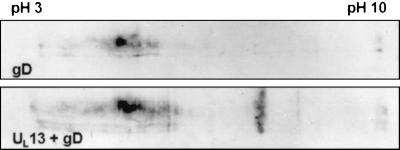
UL13 does not target gD for proteasome-dependent degradation.
The results of the experiments described above suggest that expression of the UL13 protein kinase targets US1.5 for proteasome-dependent degradation, and the question arose of whether this effect was specific to US1.5 or whether it also affected other viral proteins. To answer this question, we compared the expression of gD in cells infected with Bac-gD (44) alone or in the presence of the UL13 protein kinase. In this experiment, rabbit skin cells were infected with 10 PFU of Bac-gD alone or 10 PFU each of Bac-gD and Bac-UL13 per cell. The cultures were harvested 24 h after infection and processed as described above except that the cell lysates were probed with anti-gD monoclonal antibody. As shown in Fig. 6, simultaneous infection of cells with Bac-gD and Bac-UL13 did not have a negative effect on the accumulation of gD in these cells, suggesting that UL13 specifically targets US1.5 for proteasome-dependent degradation rather than generally activating the proteasome-dependent degradation pathway or mediating the general degradation of viral proteins.
FIG. 6.
Effect of UL13 protein kinase expression on accumulation of gD expressed via recombinant baculovirus. Replicate cultures were either mock infected or infected with approximately 10 PFU of Bac-gD alone (top) or approximately 10 PFU each of Bac-gD and Bac-UL13 (bottom) and were cultured in the presence of 10 mM sodium butyrate. Cells were harvested 24 h after baculovirus infection and solubilized in two-dimensional gel lysis solution. Two-dimensional electrophoretic separations were done as described in Materials and Methods. Electrophoretically separated proteins were transferred to nitrocellulose sheets and reacted with mouse monoclonal antibody directed against gD as described in Materials and Methods.
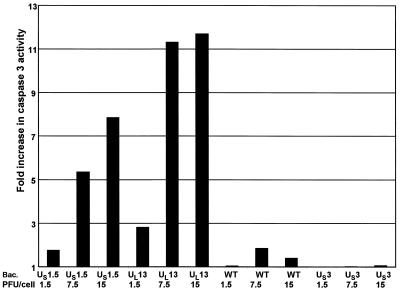
Both US1.5 and UL13 activate caspase 3 in rabbit skin cells.
The experiments presented above indicated that HSV-1 specifically targets a viral regulatory protein, US1.5, for degradation via a viral protein kinase encoded by UL13. Therefore, it was of interest to determine why this degradation event might be of advantage to the virus. As discussed in the Introduction, the d120 mutant predominately expresses α proteins and induces apoptosis (11, 21). As elimination of a cytotoxic proapoptotic factor would be advantageous and US1.5 was operationally defined as an α protein (9), the question arose of whether US1.5 induced apoptosis. To test this hypothesis, we measured DEVDase activity to assess caspase 3 activation, a seminal indicator of induction of apoptosis, in infected rabbit skin cells. The cells were harvested 24 h after infection with the indicated recombinant baculoviruses shown in Fig. 7. The results, normalized with respect to the level of caspase 3 activity in mock-infected cells, indicate that both US1.5 and UL13 protein kinases induced caspase 3 activity in a dose-dependent manner. In cells infected with wild-type baculovirus, DEVDase activity was only slightly higher than that in mock-infected cells. Cells infected with Bac-US3, which carries a recombinant gene shown to have an antiapoptotic function (28, 29), showed no significant DEVDase activity above that of mock-infected cells. These results indicate that expression of US1.5 or UL13 alone, in the absence of any other viral genes, induced apoptosis in rabbit skin cells.
FIG. 7.
Effect of baculovirus-mediated gene expression on DEVDase activity in cells. Replicate 25-cm2 flask cultures of rabbit skin cells were either mock infected or infected with the indicated PFU of Bac-US1.5, Bac-UL13, Bac-WT, or Bac-US3 per cell. The cells were harvested 24 h after baculovirus infection and assayed for DEVDase activity colorimetrically at 405 nm as described in Materials and Methods. The results are expressed as the fold increase in activity over that of mock-infected cells.
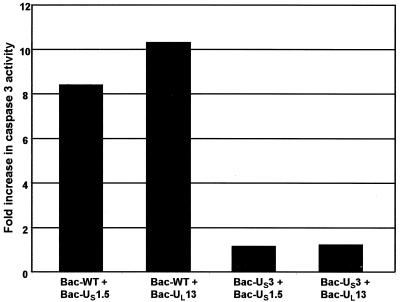
US3 blocks caspase 3 activation induced by US1.5 and UL13.
Since US3 protein kinase provided in trans has been shown earlier to block apoptosis induced by the d120 mutant (28), it was of interest to determine whether coinfection of Bac-US3 with Bac-US1.5 or Bac-UL13 blocked caspase 3 activation induced by US1.5 or UL13. In this series of experiments, DEVDase activity was measured in rabbit skin cells harvested 24 h after infection with 7.5 PFU per cell of Bac-US1.5 or Bac-UL13 and 7.5 PFU per cell of wild-type baculovirus or Bac-US3. The results shown in Fig. 8 were as follows. Cells coinfected with Bac-US1.5 and wild-type baculovirus exhibited an 8.4-fold increase in caspase 3 activity relative to that of mock-infected cells, while in cells coinfected with Bac-US1.5 and Bac-US3, the DEVDase activity was only slightly higher (1.2-fold) than that detected in mock-infected cells. Likewise, a 10-fold increase in caspase 3 activity was observed in cells coinfected with Bac-UL13 and wild-type baculovirus compared to that observed in mock-infected cells while only a 1.2-fold increase was observed in cells coinfected with Bac-UL13 and Bac-US3. These results indicate that expression of US3 blocks the activation of caspase 3 in rabbit skin cells mediated by either US1.5 or UL13 proteins.
FIG. 8.
Effect of baculovirus-mediated gene expression on DEVDase activity in cells. Replicate 25-cm2 flask cultures of rabbit skin cells were either mock infected or infected with 15 PFU of the indicated baculoviruses per cell. The cells were harvested 24 h after baculovirus infection and assayed for DEVDase activity colorimetrically at 405 nm as described in Materials and Methods. The results are expressed as fold increase in activity over that of mock-infected cells.
DISCUSSION
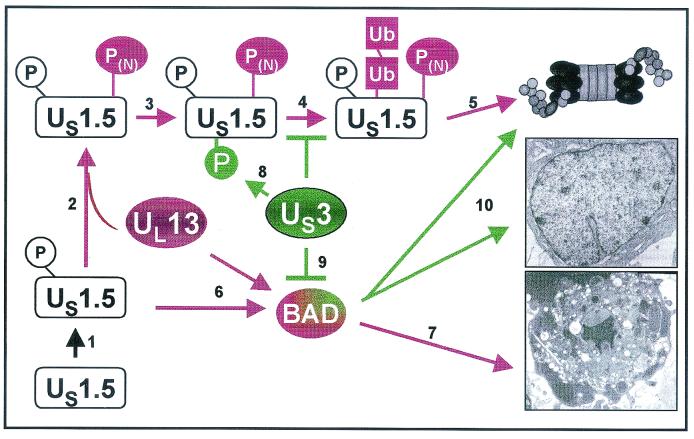
In the studies described in this report, we have examined the interplay of three HSV-1 proteins, US1.5, US3, and UL13, in the context of mammalian cells transduced to express the proteins individually or in pairs by recombinant baculoviruses. The salient features of the results are as follows. (i) The accumulation of the US1.5 protein was reduced by concurrent expression of the UL13 protein kinase and was augmented by concurrent expression of the US3 protein kinase. The magnitude of the reduction or increase in the accumulation of the US1.5 protein was cell type dependent. The effect of UL13 kinase appears to be specific inasmuch as it did not affect the accumulation of gD in cells doubly infected by recombinant baculoviruses. (ii) The reduction in the accumulation of the US1.5 protein in the presence of UL13 protein kinase was only partially due to proteasome-dependent degradation. (iii) Both US1.5 and UL13 proteins activated caspase 3, indicative of programmed cell death. (iv) Concurrent expression of US3 protein kinase blocked activation of caspase 3 mediated by both US1.5 and UL13. The interpretation of these results is tempered by the fact that in the course of viral infection there is a complex interplay between viral gene products. Although analyses of viral gene functions expressed in cells by isolated viral genes is extremely valuable, the functions expressed by isolated viral gene products may not reflect the events that occur in HSV-1-infected cells. The interpretation of the data must take into account what is actually confirmed in cells infected with wild-type or appropriate mutant viruses. A model of the functional interactions of these proteins interpreted in the context of HSV-1-infected cells is shown in Fig. 9. The key features of this model are discussed below.
FIG. 9.
Schematic representation of a model of the functions of viral proteins described in this report. The model consists of the following elements. US1.5 is rapidly phosphorylated by cellular kinases (step 1) to yield a product capable of inducing apoptosis possibly by activation of BAD (steps 6 and 7). In the presence of UL13 protein kinase, US1.5 is phosphorylated and targeted for degradation by the ubiquitin proteasomal pathway (steps 3 and 4). In the presence of US3 protein kinase, US1.5 is additionally posttranslationally modified precluding its degradation. Results published elsewhere indicate that US3 protein kinase blocks apoptosis induced by BAD (step 10) and that in the presence of the protein kinase, BAD is in part degraded. The remaining BAD is posttranslationally processed (30).
The results obtained in the system described in this report and with HSV-1-infected cells suggest that US1.5 is posttranslationally processed in the absence of either UL13 or US3 protein kinases. ICP22 and US1.5 are phosphorylated by cellular kinases (31), and at least ICP22 is also nucleotidylylated by casein kinase II (26, 27)
The results presented in this report indicate that US1.5 is in part degraded by the ubiquitin-proteasomal pathway and that it causes apoptosis in cells infected with a recombinant baculovirus expressing the protein. Partial degradation is deduced from the observation that the amounts of accumulating US1.5 protein increased in the presence of MG132, albeit not to the level observed in the presence of US3 protein kinase. A central question is the function of the two protein kinases in the accumulation of the US1.5 protein. One hypothesis that explains the data and is at least partially supported by the results of analyses of HSV-1-infected cells is that US1.5 signals the induction of apoptosis, that UL13 protein kinase regulates the accumulation of US1.5 protein by targeting it for degradation and, in the context of baculovirus-transduced cells, it induces apoptosis, and that the US3 protein kinase blocks apoptosis. While US3 expression also promotes modification of US1.5, it does not target US1.5 for degradation, suggesting that modifications of US1.5 mediated by each viral kinase have discrete functions. Accordingly, the levels of accumulated US1.5 reflect both the decrease in degradation of the protein and the continued synthesis of US1.5 in the absence of induced programmed cell death. The data in support of the hypothesis are as follows. (i) The accumulation of HSV-1 progeny reaches completion between 18 and 24 h after infection depending on cell type and is accompanied by extensive cytopathic effects. An earlier report from this laboratory showed that in HEp-2 cells overexpressing Bcl-2, the development of cytopathic effects is delayed without significant effects on viral replication (15). These results suggest that cell death at the end of the replicative cycle reflects proapoptotic events blocked by Bcl-2. Since apoptosis is not induced in wild-type virus-infected cells during the replicative phase of infection, the data indicate that the cellular environment is tightly regulated by viral gene products.
(ii) As shown in the studies reported here, the accumulation of US1.5 is cell type dependent both in HSV-1-infected cells and in cells transduced with recombinant baculoviruses. Thus, US1.5 accumulates to different extents in HSV-1-infected or transduced Vero cells and in rabbit skin cells but is barely detectable in HEp-2 cells (Fig. 2 and 4; R. Hagglund, A. P. W. Poon, and B. Roizman, unpublished results). Curiously, although the US1.5 protein meets the definition of an α gene, in wild-type HSV-1-infected cells, it accumulates late in infection at times consistent with the development of cytopathic effects related to apoptosis. In cells infected at relatively high multiplicities of infection, the accumulation of the US1.5 protein continues unabated as late as 30 h after infection (A. P. W. Poon and B. Roizman, unpublished results). Although the results indicate that US1.5 mediates the activation of caspase 3, the precise role of US1.5 in apoptosis in wild-type-virus-infected cells remains to be defined.
(iii) Both ICP22 and the US1.5 proteins are extensively posttranslationally modified by the UL13 protein kinase (references 31, 35, and 36 and Fig. 2 and 5). Evidence supporting extensive posttranslational modification is reflected in the number and charges of isoforms made in HSV-1-infected cells and in cells transduced by recombinant baculoviruses expressing US1.5 and UL13 proteins. As transduction of the viral kinases leads to the observation of highly electronegative US1.5 isoforms and addition of a phosphate group to a protein makes it more electronegative, it is likely that the viral kinases mediate the phosphorylation of the US1.5 protein. The evidence presented in Fig. 4 and 5 indicate that UL13 function targets US1.5 protein for proteasomal degradation.
Phosphorylation is a common mechanism for targeting proteins for proteasome-dependent degradation in the cell. Proteins are targeted to the 26S proteasome for degradation by the covalent addition of a multiubiquitin chain. A cascade of ubiquitylation enzymes is responsible for the activation of ubiquitin and the assembly of the polyubiquitin chain (reviewed in reference 18). Briefly, ubiquitin is activated by the E1 ubiquitin-activating enzyme and trans-esterified to the E2 ubiquitin conjugating enzyme. The E3 ubiquitin ligase binds both the E2 and substrate proteins, facilitating their interaction and the assembly of the polyubiquitin chain. E3 enzyme complexes, such as SCFβ-TrCP, SCFGrr1, SCFSkp2, and SCFCdc4, include a subunit which contains a WD40 motif which specifically binds phosphorylated target proteins (reviewed in reference 12). Thus, only phosphorylated species of the target protein are ubiquitinated and targeted for degradation. We hypothesize that phosphorylation of US1.5 targets it for interaction with an E3 ubiqutin ligase which specifically binds phosphorylated targets. US1.5 is targeted for degradation by UL13 in the absence of any other viral proteins.
(iv) Cells transduced with Bac-UL13 activated caspase 3, a finding not observed with HSV-1(F)-infected cells. As noted in the Introduction, the UL13 protein kinase has a wide substrate range which may include cellular protein essential for cellular survival. This substrate may be unavailable in HSV-1-infected cells or its modification by UL13 or induction of apoptosis after such modification is blocked by other viral proteins, such as US3. Thus, the UL13 protein kinase encodes functions which promote apoptosis and impede apoptosis induced by US1.5 by effecting its degradation. A possible reason that the dual mechanism of regulation of US1.5 activity might be advantageous for the virus is that US3 might not be as effective in inhibiting induction of apoptosis by US1.5 at high doses. While some degree of US1.5 expression may be necessary for functions such as the expression of a subset of γ2 genes (31, 35), amounts of US1.5 protein above what is necessary for these functions would not be advantageous for the virus because of its ability to induce apoptosis. Thus, the virus tightly regulates US1.5 accumulation.
(v) As noted in the Introduction, US3 protein kinase mediates the phosphorylation of ICP22 and of US1.5, but the identity of its substrates is not known (reference 36 and Fig. 4 and 5). Recent studies have extended the early observations that US3 blocks apoptosis in d120 mutant-infected cells by demonstrating that it acts at a premitochondrial stage (28). Finally, still more recent studies demonstrated that US3 protein kinase blocks apoptosis induced by BAD. Thus, in cells transduced by BAD and US3 ORFs, BAD is partially degraded and what remains is posttranslationally modified (29). In this study, we show that US3 blocked apoptosis induced by the recombinant baculoviruses expressing either US1.5 or UL13 proteins. As illustrated in Fig. 9, it is likely that both US1.5 and UL13 proteins induce apoptosis at a similar, premitochondrial stage and that the US3 protein kinase blocks apoptosis by interfering with the activation of BAD protein.
To date, at least four different mutants have been shown to induce apoptosis. These are a mutant lacking gD (44), mutants lacking the regulatory proteins ICP4 (21) or ICP27 (5, 6), and a temperature-sensitive mutant unable to release viral DNA at nuclear pores from capsids at the nonpermissive temperature (14). The results presented in this and earlier reports from this laboratory would predict that US1.5 protein expressed with α kinetics may play a role in the induction of apoptosis in cells infected with mutants lacking regulatory proteins but not in cells exposed to the mutants lacking gD or that are incapable of releasing viral DNA from capsids. In the course of wild-type virus infection, induction of apoptosis by the US1.5 protein would be inhibited by UL13 and US3 functions discussed above. Conversely, the absence of the two kinases would not automatically lead to apoptosis inasmuch as expression of the US1.5 gene appears to be cell type dependent and no evidence has emerged so far that indicates that ΔUL13/ΔUS3 or ΔUL13 recombinant viruses induce programmed cell death. In essence, while we have demonstrated that US1.5 expression leads to apoptosis in rabbit skin cells in the absence of other viral proteins, cellular functions in response to stress may block this effect.
Apoptosis has been shown to be a common host cell response to viral infection (reviewed in reference 43). This response is advantageous to the host because the premature death of infected cells checks the production of progeny virus, thus impairing the spread of the virus to other cells. Many viruses encode functions which block apoptosis induced by replicative functions of the virus (21, 37, 38, 45). Current evidence indicates that HSV-1 has evolved multiple mechanisms to thwart this cellular response to its replicative functions.
Acknowledgments
These studies were aided by grants from the National Cancer Institute (CA87761, CA83939, CA71933, and CA78766), the United States Public Health Service. R.H. is a Howard Hughes Medical Institute Predoctoral Fellow.
REFERENCES
- 1.Ackermann, M., M. Sarmiento, and B. Roizman. 1985. Application of antibody to synthetic peptides for characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J. Virol. 56:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in the activity of the G2/M phase cellular kinase cdc2 in herpes simplex virus 1-infected cells requires the expression of α22/US1.5 and UL13 viral genes. J. Virol. 74:8–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, S., T. Honda, F. Goshima, D. Wantanbe, Y. Miyake, Y. Sugiura, and Y. Nishiyama. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80:51–56. [DOI] [PubMed] [Google Scholar]
- 4.Asano, S., T. Honda, F. Goshima, Y. Nishiyama, and Y. Sugiura. 2000. US3 protein kinase of herpes simplex virus protects primary afferent neurons from virus-induced apoptosis in ICR mice. Neurosci. Lett. 294:105–108. [DOI] [PubMed] [Google Scholar]
- 5.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for prevention of apoptosis in infected human cells. J. Virol. 73:2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert, M., J. O’Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkelman, T., and T. Stenstedt. 1998. 2-D electrophoresis. Using immobilized pH gradients. Principles and methods. Amersham Pharmacia Biotech, Piscataway, N.J.
- 8.Bjellqvist, B., K. Ek, P. G. Righetti, E. Gianazza, A. Görg, R. Westermeier, and W. Postel. 1982. Isoelectric focusing in immobilized pH gradients: principle, methodology, and some applications. J. Biochem. Biophys. Methods 6:317–339. [DOI] [PubMed] [Google Scholar]
- 9.Carter, K. L., and B. Roizman. 1996. The promoter and transcriptional unit of a novel herpes simplex virus 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J. Virol. 70:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condreay, J. P., S. M. Witherspoon, W. C. Clay, and T. A. Kost. 1999. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA 96:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshaies, R. J. 1999. SCF and cullin/ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15:435–467. [DOI] [PubMed] [Google Scholar]
- 13.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J. Gen. Virol. 2:357–364. [DOI] [PubMed] [Google Scholar]
- 14.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvan, V., R. Brandimarti, J. Munger, and B. Roizman. 2000. Bcl-2 blocks a caspase-dependent pathway of apoptosis activated by herpes simplex virus 1 infection in HEp-2 cells. J. Virol. 74:1931–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson, P. K., A. G. Eldridge, E. Freed, L. Furstenthal, J. Y. Hsu, B. K. Kaiser, and J. D. R. Reimann. 2000. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10:429–439. [DOI] [PubMed] [Google Scholar]
- 19.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H.-Y. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, US5 and US3. J. Virol. 73:8950–8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1δ is hyper-phosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J. Virol. 72:1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leopardi, R., and B. Roizman. 1996. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583–9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leopardi, R., P. L. Ward, W. O. Ogle, and B. Roizman. 1997. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the UL13 protein kinase. J. Virol. 71:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackem, S., and B. Roizman. 1980. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of α genes. Proc. Natl. Acad. Sci. USA 77:7122–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251–254. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell, C., J. A. Blaho, and B. Roizman. 1994. Casein kinase II specifically nucleotidylylates in vitro the amino acid sequence of the protein encoded by the α22 gene of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 91:11864–11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell, C., J. A. Blaho, L. McCormick, and B. Roizman. 1997. The nucleotidylylation of herpes simplex virus 1 regulatory protein α22 by human casein kinase II. J. Biol. Chem. 272:25394–25400. [DOI] [PubMed] [Google Scholar]
- 28.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37–48. [DOI] [PubMed] [Google Scholar]
- 31.Ogle, W. O., and B. Roizman. 1999. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J. Virol. 73:4305–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon, A. P. W., W. O. Ogle, and B. Roizman. 2000. Posttranslational processing of infected cell protein 22 mediated by viral protein kinases is sensitive to amino acid substitutions at distant sites and can be cell-type specific. J. Virol. 74:11210–11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post, L. E., and B. Roizman. 1981. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell 25:227–232. [DOI] [PubMed] [Google Scholar]
- 34.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for growth in cell culture. J. Virol. 61:2896–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein α22 meditated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc. Natl. Acad. Sci. USA 89:7310–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao, L., M. Debbas, P. Sabbatini, D. Hockenbery, S. Korsmeyer, and E. White. 1992. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc. Natl. Acad. Sci. USA 89:7742–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray, C. A., R. A. Black, S. R. Kronheim, T. A. Greenstreet, P. R. Sleath, G. S. Salvesen, and D. J. Pickup. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69:597–604. [DOI] [PubMed] [Google Scholar]
- 39.Rixon, F. J., and J. B. Clements. 1982. Detailed structural analysis of two spliced HSV-1 immediate-early mRNAs. Nucleic Acids Res. 10:2241–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roizman, B., and D. M. Knipe. 2001. The replication of herpes simplex viruses, p.2399–;2459. In D. M. Knipe, P. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Williams and Wilkins, New York, N.Y.
- 41.Sears, A. E., I. W. Halliburton, B. Meignier, S. Silver, and B. Roizman. 1985. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cell lines and establishment of latency in mice. J. Virol. 55:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson, R. J., M. Sullivan, and G. F. Vande Woude. 1981. Structures of two spliced herpes simplex virus type 1 immediate-early mRNAs which map at the junctions of the unique and reiterated regions of the virus DNA S component. J. Virol. 37:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, L. S., C. W. Dawson, and A. G. Eliopoulos. 1997. Viruses and apoptosis. Br. Med. Bull. 53:509–521. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]