Abstract
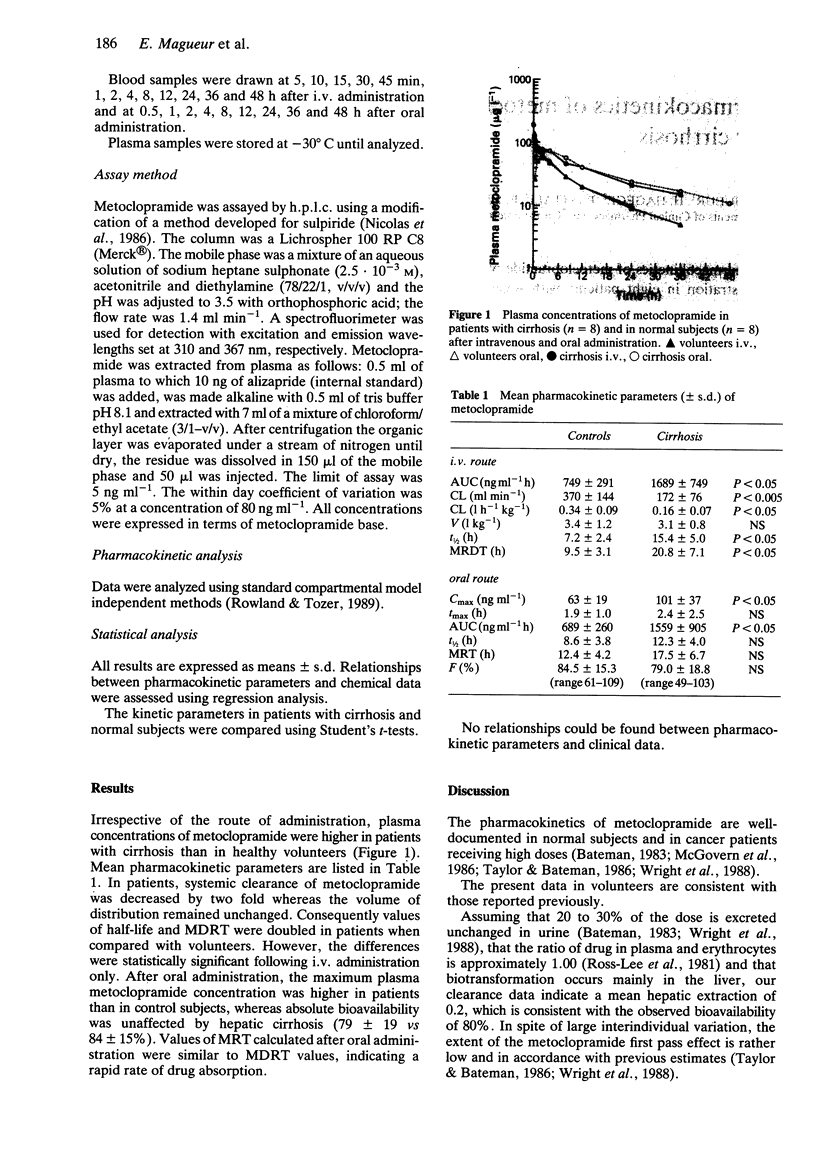
The pharmacokinetics of metoclopramide were investigated after intravenous and oral administration in eight patients with severe alcoholic cirrhosis and in eight healthy volunteers. As a consequence of a 50% lower clearance (0.16 +/- 0.07 vs 0.34 +/- 0.09 l h-1 kg-1, plasma drug concentrations and the half-life of metoclopramide were greater in patients following both routes of drug administration. Volume of distribution (3.1 +/- 0.8 vs 3.4 +/- 1.2 l kg-1) and absolute bioavailability (79 +/- 19 vs 84 +/- 15%) were similar in the two groups. The adverse effects of metoclopramide observed in patients with marked hepatic impairment are likely to result from increased accumulation of the drug as a result of impaired clearance. Consequently a reduction in dose of 50% is recommended in patients with severe liver cirrhosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman D. N. Clinical pharmacokinetics of metoclopramide. Clin Pharmacokinet. 1983 Nov-Dec;8(6):523–529. doi: 10.2165/00003088-198308060-00003. [DOI] [PubMed] [Google Scholar]
- Bateman D. N., Gokal R., Dodd T. R., Blain P. G. The pharmacokinetics of single doses of metoclopramide in renal failure. Eur J Clin Pharmacol. 1981;19(6):437–441. doi: 10.1007/BF00548588. [DOI] [PubMed] [Google Scholar]
- Bernardi M., Trevisani F., Gasbarrini G. Metoclopramide administration in advanced liver disease. Gastroenterology. 1986 Aug;91(2):523–523. doi: 10.1016/0016-5085(86)90610-4. [DOI] [PubMed] [Google Scholar]
- D'Arienzo A., Ambrogio G., Di Siervi P., Perna E., Squame G., Mazzacca G. A randomized comparison of metoclopramide and domperidone on plasma aldosterone concentration and on spironolactone-induced diuresis in ascitic cirrhotic patients. Hepatology. 1985 Sep-Oct;5(5):854–857. doi: 10.1002/hep.1840050524. [DOI] [PubMed] [Google Scholar]
- Dawling S., Crome P. Clinical pharmacokinetic considerations in the elderly. An update. Clin Pharmacokinet. 1989 Oct;17(4):236–263. doi: 10.2165/00003088-198917040-00003. [DOI] [PubMed] [Google Scholar]
- Harrington R. A., Hamilton C. W., Brogden R. N., Linkewich J. A., Romankiewicz J. A., Heel R. C. Metoclopramide. An updated review of its pharmacological properties and clinical use. Drugs. 1983 May;25(5):451–494. doi: 10.2165/00003495-198325050-00002. [DOI] [PubMed] [Google Scholar]
- Howden C. W., Birnie G. G., Brodie M. J. Drug metabolism in liver disease. Pharmacol Ther. 1989;40(3):439–474. doi: 10.1016/0163-7258(89)90088-0. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Wilting J., Janssen L. H. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol Rev. 1988 Mar;40(1):1–47. [PubMed] [Google Scholar]
- Lehmann C. R., Heironimus J. D., Collins C. B., O'Neil T. J., Pierson W. P., Crowe J. T., Melikian A. P., Wright G. J. Metoclopramide kinetics in patients with impaired renal function and clearance by hemodialysis. Clin Pharmacol Ther. 1985 Mar;37(3):284–289. doi: 10.1038/clpt.1985.41. [DOI] [PubMed] [Google Scholar]
- McGovern E. M., Grevel J., Bryson S. M. Pharmacokinetics of high-dose metoclopramide in cancer patients. Clin Pharmacokinet. 1986 Nov-Dec;11(6):415–424. doi: 10.2165/00003088-198611060-00001. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Fauvelle F., Ennachachibi A., Merdjan H., Petitjean O. Improved determination of sulpiride in plasma by ion-pair liquid chromatography with fluorescence detection. J Chromatogr. 1986 Sep 5;381(2):393–400. doi: 10.1016/s0378-4347(00)83605-1. [DOI] [PubMed] [Google Scholar]
- Ross-Lee L. M., Eadie M. J., Hooper W. D., Bochner F. Single-dose pharmacokinetics of metoclopramide. Eur J Clin Pharmacol. 1981;20(6):465–471. doi: 10.1007/BF00542101. [DOI] [PubMed] [Google Scholar]
- Taylor W. B., Bateman D. N. Oral bioavailability of high-dose metoclopramide. Eur J Clin Pharmacol. 1986;31(1):41–44. doi: 10.1007/BF00870983. [DOI] [PubMed] [Google Scholar]
- Uribe M., Ballesteros A., Strauss R., Rosales J., Garza J., Villalobos A., Briones A., Garcia Ramos G. Successful administration of metoclopramide for the treatment of nausea in patients with advanced liver disease. A double-blind controlled trial. Gastroenterology. 1985 Mar;88(3):757–762. doi: 10.1016/0016-5085(85)90147-7. [DOI] [PubMed] [Google Scholar]
- Webb D., Buss D. C., Fifield R., Bateman D. N., Routledge P. A. The plasma protein binding of metoclopramide in health and renal disease. Br J Clin Pharmacol. 1986 Mar;21(3):334–336. doi: 10.1111/j.1365-2125.1986.tb05201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M. R., Axelson J. E., Rurak D. W., McErlane B., McMorland G. H., Ongley R. C., Tam Y. K., Price J. D. Linearity of metoclopramide kinetics at doses of 5-20 mg. Br J Clin Pharmacol. 1988 Oct;26(4):469–473. doi: 10.1111/j.1365-2125.1988.tb03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


