Abstract
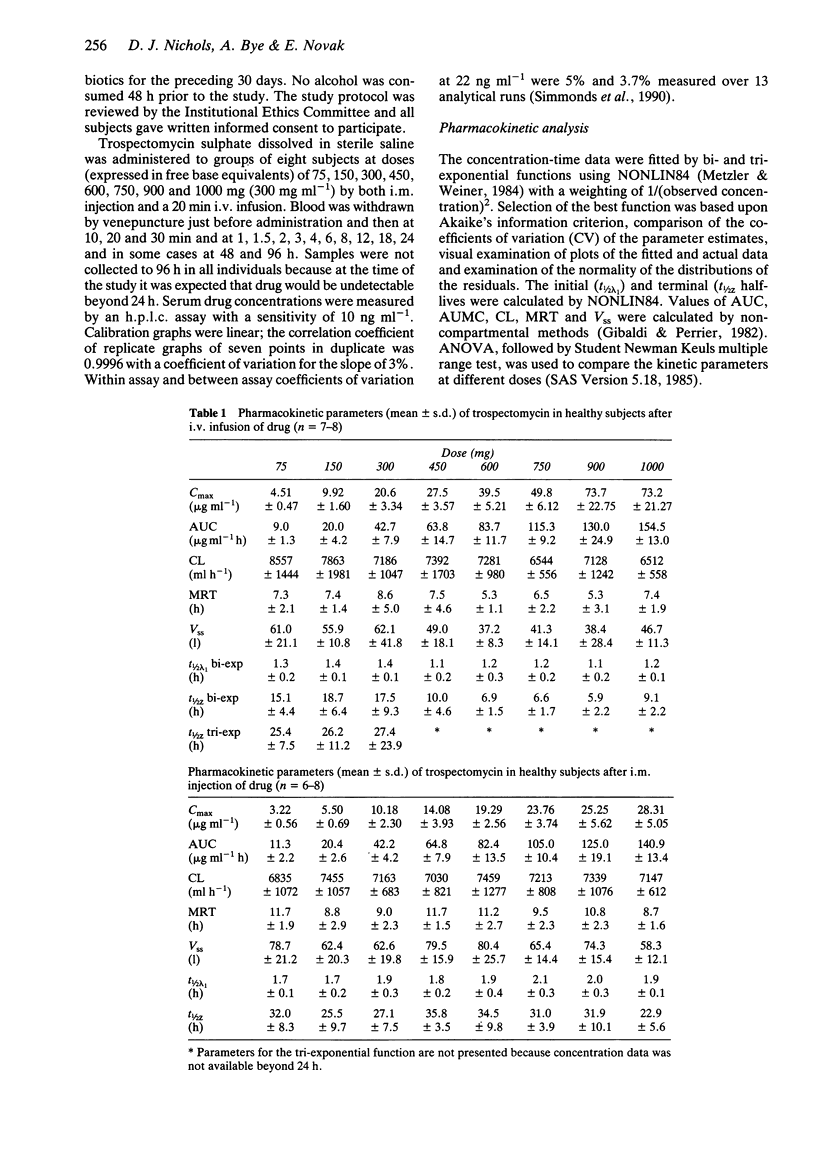
The pharmacokinetics of trospectomycin (75-1000 mg free base equivalents) were studied in 128 healthy males (eight per dose group), after a 20 min intravenous (i.v.) infusion and intramuscular (i.m.) injection of trospectomycin sulphate. The concentrations of trospectomycin in serum were described by bi- or tri-exponential disposition functions indicating an initial half-life of 1.1-1.4 h for the i.v. dose and 1.6-2.1 h for the i.m. dose and terminal half-lives of over 15 h. Most of the drug was eliminated rapidly (mean residence time 5-12 h). The distribution volume was 59-112% of body weight and clearance was 112-152 ml min-1. The absorption into blood after i.m. dosing was rapid. The area under the concentration-time curve and maximum concentration values were linearly related to dose. Serum drug concentrations fell below the minimum inhibitory concentration values for a variety of organisms by 8-12 h, which indicates that two or three times daily dosing would be appropriate. However, the long terminal half-life suggests that significant accumulation is likely in some tissues with an 8 h dose interval and this may prolong the action of trospectomycin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox J. W., Dring L. G., Ginsberg L. C., Larson P. G., Constable D. A., Ulrich R. G. Distribution and disposition of trospectomycin sulfate in the in vivo rat, perfused rat liver, and cultured rat hepatocytes. Drug Metab Dispos. 1990 Sep-Oct;18(5):726–731. [PubMed] [Google Scholar]
- Cox J. W., Ulrich R. G., Wynalda M. A., McKenna R., Larsen E. R., Ginsberg L. C., Epps D. E. Reversible, hepatic, lysosomal phospholipidosis in rat induced by subchronic daily administration of trospectomycin sulfate. Biochem Pharmacol. 1989 Oct 15;38(20):3535–3541. doi: 10.1016/0006-2952(89)90125-1. [DOI] [PubMed] [Google Scholar]
- Ulrich R. G., Petrella D. K., Larsen E. R., Cox J. W., Cramer C. T., Piper R. C., Gray J. E. Hepatic changes produced by 30-day administration of a novel aminocyclitol antibiotic, trospectomycin sulfate, to laboratory animals. Fundam Appl Toxicol. 1990 Jan;14(1):60–70. doi: 10.1016/0272-0590(90)90231-8. [DOI] [PubMed] [Google Scholar]
- Zurenko G. E., Ford C. W., Novak E. Trospectomycin, a novel spectinomycin analogue: antibacterial activity and preliminary human pharmacokinetics. Drugs Exp Clin Res. 1988;14(6):403–409. [PubMed] [Google Scholar]


