Abstract
1. We have measured plasma concentrations of alpha 1-acid glycoprotein (AGP) in 18 healthy children and 85 children with falciparum malaria in Malawi. In addition, we determined the degree of protein binding of quinine (QN) in the plasma of 52 of the patients and each of the healthy controls. 2. The mean plasma AGP concentration was higher in patients than in controls (P less than 0.0001) and remained elevated 3 weeks after complete resolution of malaria infection. 3. The mean unbound QN fraction was significantly less (P less than 0.00001) in patients with malaria (0.128 +/- 0.037) than in controls (0.193 +/- 0.051) and significantly higher (P = 0.02) in convalescence (0.153 +/- 0.067) than during acute illness. 4. There were highly significant negative correlations between plasma AGP concentration and the free QN fraction in spiked plasma samples (r = -0.534, P less than 0.0001, n = 93) and in clinical samples (r = -0.484, P less than 0.00001, n = 225). There was a significant positive correlation between plasma concentrations of AGP and another acute phase reactant, C reactive protein (P less than 0.001).
Full text
PDF

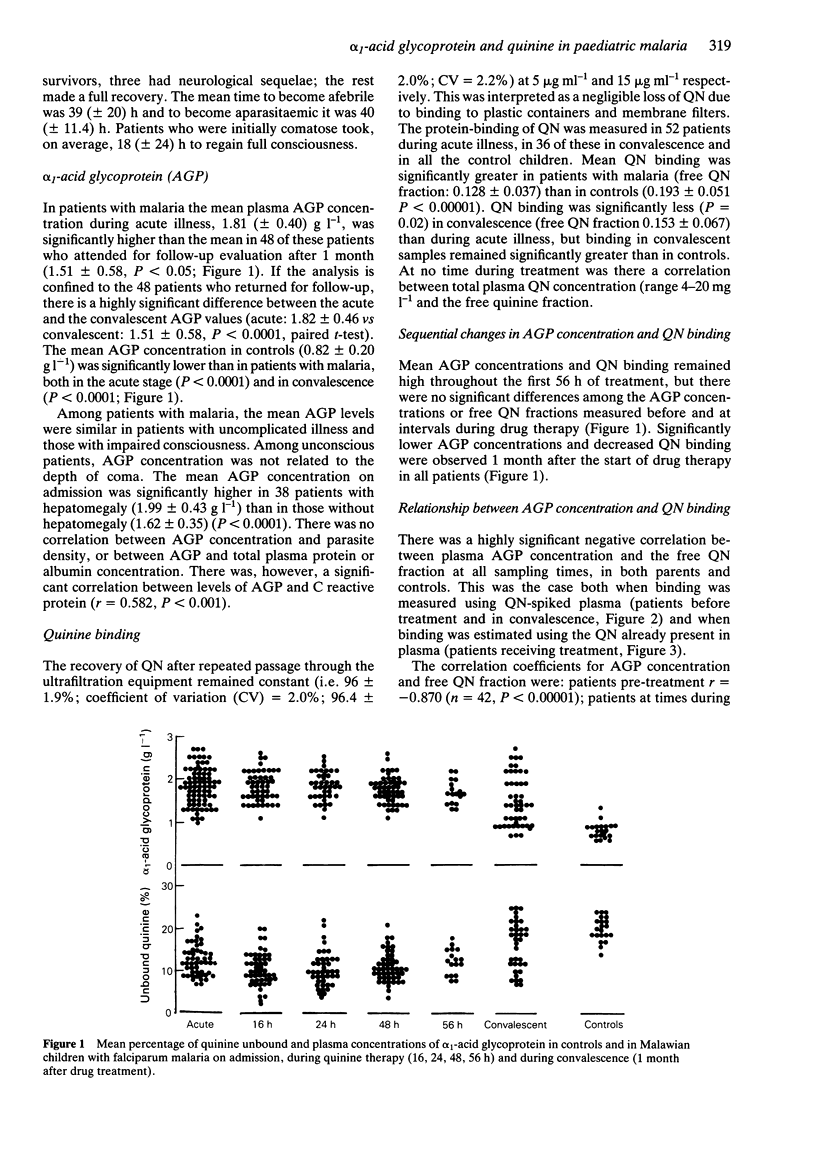
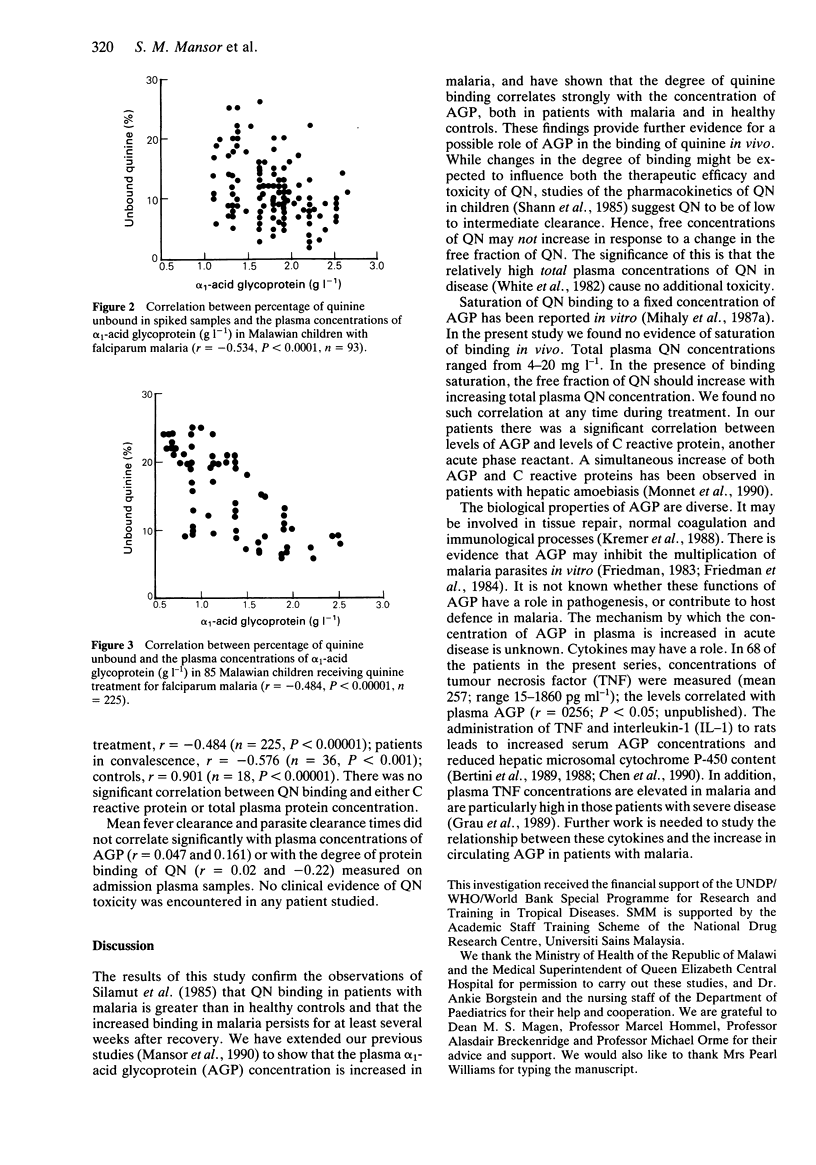

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertini R., Bianchi M., Erroi A., Villa P., Ghezzi P. Dexamethasone modulation of in vivo effects of endotoxin, tumor necrosis factor, and interleukin-1 on liver cytochrome P-450, plasma fibrinogen, and serum iron. J Leukoc Biol. 1989 Sep;46(3):254–262. doi: 10.1002/jlb.46.3.254. [DOI] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Villa P., Ghezzi P. Depression of liver drug metabolism and increase in plasma fibrinogen by interleukin 1 and tumor necrosis factor: a comparison with lymphotoxin and interferon. Int J Immunopharmacol. 1988;10(5):525–530. doi: 10.1016/0192-0561(88)90069-0. [DOI] [PubMed] [Google Scholar]
- Friedman M. J., Blankenberg T., Sensabaugh G., Tenforde T. S. Recognition and invasion of human erythrocytes by malarial parasites: contribution of sialoglycoproteins to attachment and host specificity. J Cell Biol. 1984 May;98(5):1672–1677. doi: 10.1083/jcb.98.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. J. Control of malaria virulence by alpha 1-acid glycoprotein (orosomucoid), an acute-phase (inflammatory) reactant. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5421–5424. doi: 10.1073/pnas.80.17.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Wilting J., Janssen L. H. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol Rev. 1988 Mar;40(1):1–47. [PubMed] [Google Scholar]
- Mansor S. M., Taylor T. E., McGrath C. S., Edwards G., Ward S. A., Wirima J. J., Molyneux M. E. The safety and kinetics of intramuscular quinine in Malawian children with moderately severe falciparum malaria. Trans R Soc Trop Med Hyg. 1990 Jul-Aug;84(4):482–487. doi: 10.1016/0035-9203(90)90007-2. [DOI] [PubMed] [Google Scholar]
- Mihaly G. W., Ching M. S., Klejn M. B., Paull J., Smallwood R. A. Differences in the binding of quinine and quinidine to plasma proteins. Br J Clin Pharmacol. 1987 Dec;24(6):769–774. doi: 10.1111/j.1365-2125.1987.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly G. W., Hyman K. M., Smallwood R. A., Hardy K. J. High-performance liquid chromatographic analysis of quinine and its diastereoisomer quinidine. J Chromatogr. 1987 Mar 20;415(1):177–182. doi: 10.1016/s0378-4347(00)83207-7. [DOI] [PubMed] [Google Scholar]
- Molyneux M. E., Taylor T. E., Wirima J. J., Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989 May;71(265):441–459. [PubMed] [Google Scholar]
- Monnet D., Kokora G., Cisse M., Ferly-Therizol M., Moreau J., Durand G., Lonsdorfer A., Yapo A. E. Evaluation du processus inflammatoire dans les atteintes hépatiques: dosage immunochimique de la protéine C alpha-réactive et de l'alpha 1-glycoprotéine acide. Bull Soc Pathol Exot. 1990;83(1):107–113. [PubMed] [Google Scholar]
- Routledge P. A. The plasma protein binding of basic drugs. Br J Clin Pharmacol. 1986 Nov;22(5):499–506. doi: 10.1111/j.1365-2125.1986.tb02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F., Stace J., Edstein M. Pharmacokinetics of quinine in children. J Pediatr. 1985 Mar;106(3):506–510. doi: 10.1016/s0022-3476(85)80692-2. [DOI] [PubMed] [Google Scholar]
- Silamut K., White N. J., Looareesuwan S., Warrell D. A. Binding of quinine to plasma proteins in falciparum malaria. Am J Trop Med Hyg. 1985 Jul;34(4):681–686. doi: 10.4269/ajtmh.1985.34.681. [DOI] [PubMed] [Google Scholar]
- Voulgari F., Cummins P., Gardecki T. I., Beeching N. J., Stone P. C., Stuart J. Serum levels of acute phase and cardiac proteins after myocardial infarction, surgery, and infection. Br Heart J. 1982 Oct;48(4):352–356. doi: 10.1136/hrt.48.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J., Looareesuwan S., Warrell D. A., Warrell M. J., Bunnag D., Harinasuta T. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated Falciparum malaria. Am J Med. 1982 Oct;73(4):564–572. doi: 10.1016/0002-9343(82)90337-0. [DOI] [PubMed] [Google Scholar]


