Abstract
1. A genetic polymorphism in human erythrocyte thiopurine methyltransferase activity (RBC TPMT) resulting in a trimodal phenotypic distribution has been demonstrated both in a North American population and in British children. 2. We studied whether such a polymorphism may be also present in a white French population by testing RBC TPMT activity in 303 randomly selected blood donors. 3. We found a large inter-individual variation in RBC TPMT activity which ranged from 2 to 40 nmol ml-1 packed RBC h-1, with a mean value of 15.4 +/- 7.0 nmol ml-1 packed RBC h-1. The enzyme activity was not significantly influenced by the sex and age of the subjects. 4. In our population sample, we found no subject with undetectable enzyme activity. However, the probit plot of the log RBC TPMT activity showed a highly significant change in slope at a TPMT activity of 7.5 nmol ml-1 packed RBC h-1. Thirty four subjects (11% of our population) had TPMT activities below 7.5 nmol ml-1 packed RBC h-1. 5. These data are consistent with the view that the genetic polymorphism of TPMT activity described in populations from North America and the United Kingdom is also present in a French population, with about 89% of subjects exhibiting a high activity and 11% an intermediate activity.
Full text
PDF


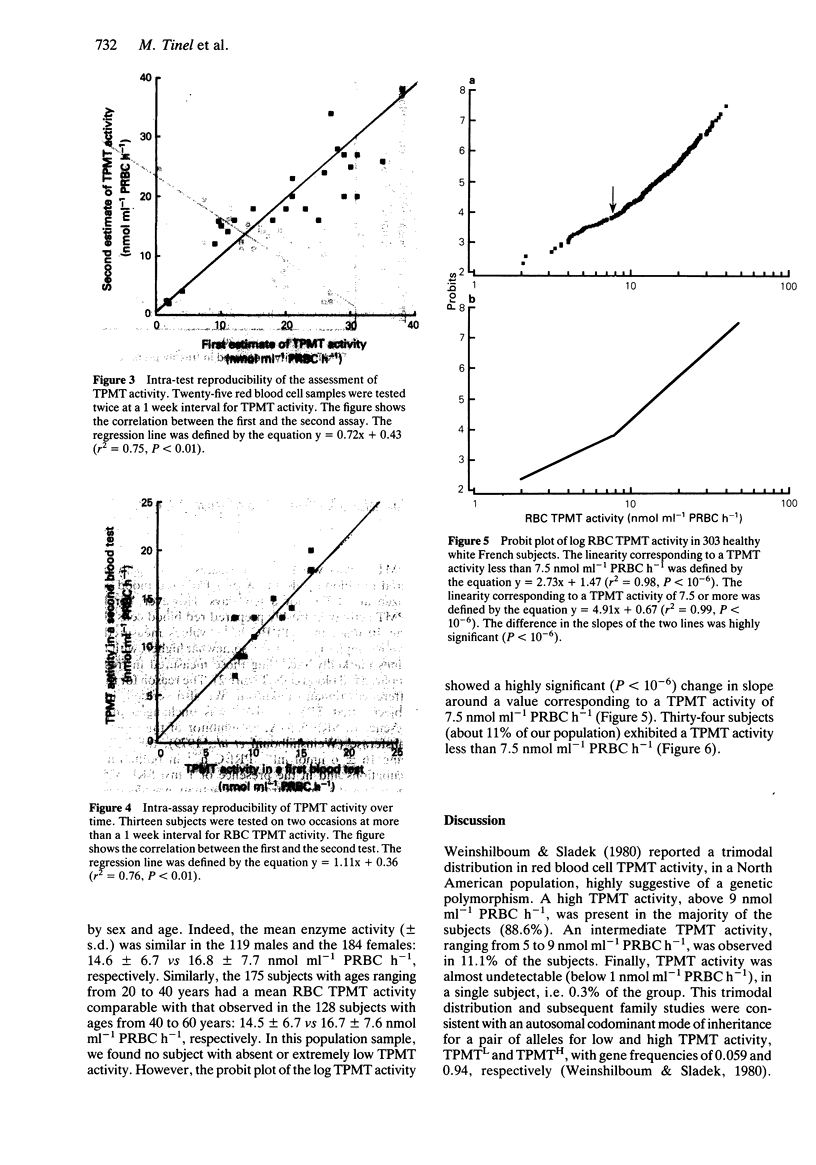
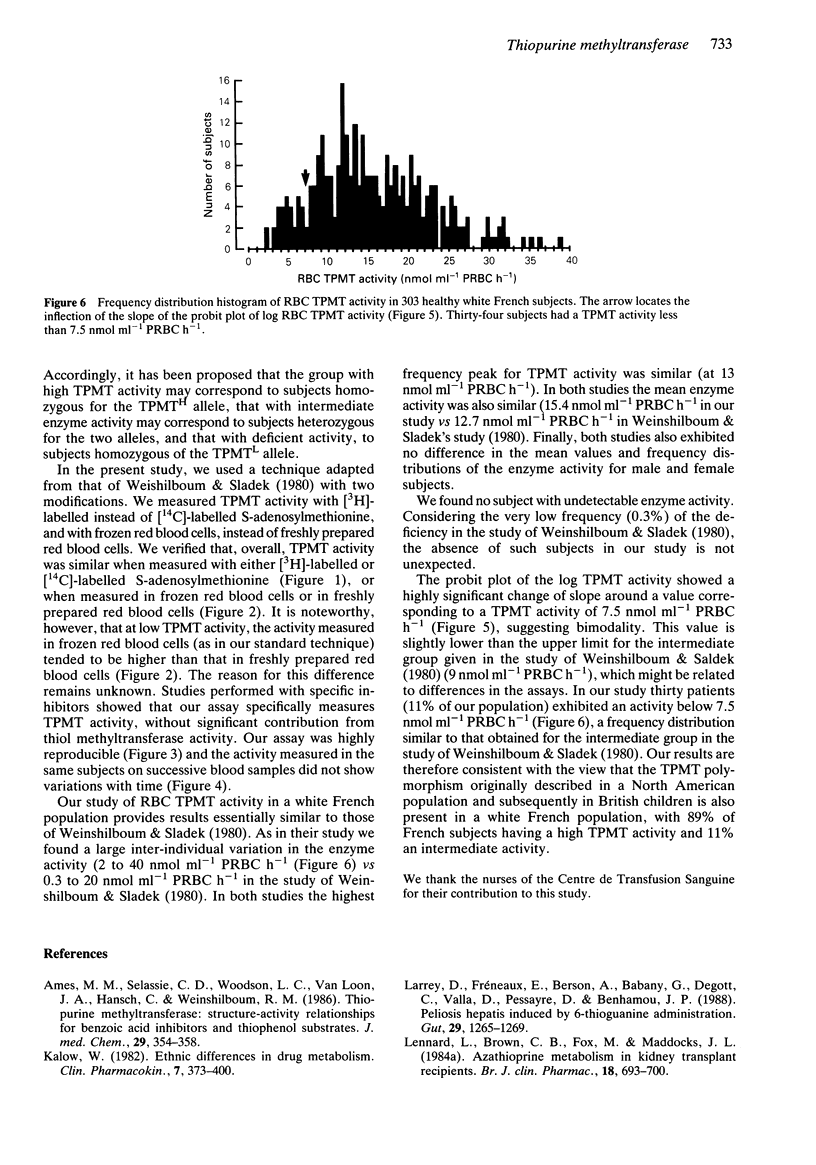

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames M. M., Selassie C. D., Woodson L. C., Van Loon J. A., Hansch C., Weinshilboum R. M. Thiopurine methyltransferase: structure-activity relationships for benzoic acid inhibitors and thiophenol substrates. J Med Chem. 1986 Mar;29(3):354–358. doi: 10.1021/jm00153a009. [DOI] [PubMed] [Google Scholar]
- Kalow W. Ethnic differences in drug metabolism. Clin Pharmacokinet. 1982 Sep-Oct;7(5):373–400. doi: 10.2165/00003088-198207050-00001. [DOI] [PubMed] [Google Scholar]
- Larrey D., Fréneaux E., Berson A., Babany G., Degott C., Valla D., Pessayre D., Benhamou J. P. Peliosis hepatis induced by 6-thioguanine administration. Gut. 1988 Sep;29(9):1265–1269. doi: 10.1136/gut.29.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Brown C. B., Fox M., Maddocks J. L. Azathioprine metabolism in kidney transplant recipients. Br J Clin Pharmacol. 1984 Nov;18(5):693–700. doi: 10.1111/j.1365-2125.1984.tb02531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Harrington C. I., Wood M., Maddocks J. L. Metabolism of azathioprine to 6-thioguanine nucleotides in patients with pemphigus vulgaris. Br J Clin Pharmacol. 1987 Feb;23(2):229–233. doi: 10.1111/j.1365-2125.1987.tb03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S., Van Loon J., Weinshilboum R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990 Jul 28;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- Lennard L., Murphy M. F., Maddocks J. L. Severe megaloblastic anaemia associated with abnormal azathioprine metabolism. Br J Clin Pharmacol. 1984 Feb;17(2):171–172. doi: 10.1111/j.1365-2125.1984.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Rees C. A., Lilleyman J. S., Maddocks J. L. Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol. 1983 Oct;16(4):359–363. doi: 10.1111/j.1365-2125.1983.tb02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Thomas S., Harrington C. I., Maddocks J. L. Skin cancer in renal transplant recipients is associated with increased concentrations of 6-thioguanine nucleotide in red blood cells. Br J Dermatol. 1985 Dec;113(6):723–729. doi: 10.1111/j.1365-2133.1985.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Weinshilboum R. M. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989 Aug;46(2):149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- Otterness D. M., Keith R. A., Weinshilboum R. M. Thiopurine methyltransferase: mouse kidney and liver assay conditions, biochemical properties and strain variation. Biochem Pharmacol. 1985 Nov 1;34(21):3823–3830. doi: 10.1016/0006-2952(85)90430-7. [DOI] [PubMed] [Google Scholar]
- Otterness D. M., Weinshilboum R. M. Mouse thiopurine methyltransferase pharmacogenetics: monogenic inheritance. J Pharmacol Exp Ther. 1987 Mar;240(3):817–824. [PubMed] [Google Scholar]
- REMY C. N. Metabolism of thiopyrimidines and thiopurines. S-Methylation with S-adenosylmethionine transmethylase and catabolism in mammalian tissues. J Biol Chem. 1963 Mar;238:1078–1084. [PubMed] [Google Scholar]
- Raymond F. A., Weinshilboum R. M. Microassay of human erythrocyte catechol-O-methyltransferase: removal of inhibitory calcium ion with chelating resin. Clin Chim Acta. 1975 Jan 20;58(2):185–194. doi: 10.1016/s0009-8981(75)80012-x. [DOI] [PubMed] [Google Scholar]
- Van Loon J. A., Weinshilboum R. M. Thiopurine methyltransferase biochemical genetics: human lymphocyte activity. Biochem Genet. 1982 Aug;20(7-8):637–658. doi: 10.1007/BF00483962. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A., Pazmiño P. A. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978 May 2;85(3):323–333. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S., Klumpp S. Human erythrocyte thiol methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1979 Sep 15;97(1):59–71. doi: 10.1016/0009-8981(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Woodson L. C., Ames M. M., Selassie C. D., Hansch C., Weinshilboum R. M. Thiopurine methyltransferase. Aromatic thiol substrates and inhibition by benzoic acid derivatives. Mol Pharmacol. 1983 Nov;24(3):471–478. [PubMed] [Google Scholar]
- Woodson L. C., Dunnette J. H., Weinshilboum R. M. Pharmacogenetics of human thiopurine methyltransferase: kidney-erythrocyte correlation and immunotitration studies. J Pharmacol Exp Ther. 1982 Jul;222(1):174–181. [PubMed] [Google Scholar]
- Woodson L. C., Weinshilboum R. M. Human kidney thiopurine methyltransferase. Purification and biochemical properties. Biochem Pharmacol. 1983 Mar 1;32(5):819–826. doi: 10.1016/0006-2952(83)90582-8. [DOI] [PubMed] [Google Scholar]


