Abstract
We tested whether adults (Experiment 1) and 4–5-year-old children (Experiment 2) identify the sex of high attractive faces faster and more accurately than low attractive faces in a reaction time task. We also assessed whether facial masculinity/femininity facilitated identification of sex. Results showed that attractiveness facilitated adults’ sex classification of both female and male faces and children’s sex classification of female, but not male, faces. Moreover, attractiveness affected the speed and accuracy of sex classification independent of masculinity/femininity. High masculinity in male faces, but not high femininity in female faces, also facilitated sex classification for both adults and children. These findings provide important new data on how the facial cues of attractiveness and masculinity/femininity contribute to the task of sex classification and provide evidence for developmental differences in how adults and children use these cues. Additionally, these findings provide support for Langlois and Roggman’s (1990) averageness theory of attractiveness.
Keywords: sex classification, attractiveness, masculinity, femininity, face perception
How do we identify the sex of faces? Although anthropological and experimental research shows that adult male and female faces clearly differ on many characteristics including eyes, nose size and shape, brow prominence, face shape, skin texture, and hair length, no one of these feature can be used to discriminate reliably between females and males (Brown & Perrett, 1993; Bruce et al., 1993; Enlow, 1982; Intons-Peterson, 1988; Roberts & Bruce, 1988; Yamaguchi, Hirukawa, & Kanazawa, 1995). Rather, the entire pattern or configuration of the face seems to be more important than individual features alone in the identification of sex (Brown & Perrett, 1993; Burton, Bruce, & Dench, 1993; Campanella, Chrysochoos, & Bruyer, 2001; Wild et al., 2000).
What information might aid in the identification of faces in general and sex of face in particular? One possibility is how attractive a face appears. Even young infants look longer at attractive versus unattractive Caucasian female faces, and these visual preferences generalize to female faces of other ethnic groups and infant faces, although evidence for visual preferences for male faces is mixed (Langlois et al., 2000; Langlois, Ritter, Roggman, & Vaughn, 1991; Langlois et al., 1987; Ramsey, 2003; Samuels & Ewy, 1985). Averageness theory proposes that attractive faces represent the central tendency of the population from which they are drawn: They are average in facial configuration (although not in attractiveness), they are prototypic, and, therefore, they are better examples of faces and thus preferred (Langlois & Roggman, 1990; Langlois, Roggman, & Musselman, 1994; Rhodes, Jeffery, Watson, Clifford, & Nakayama, 2003; Rhodes, Sumich, & Byatt, 1999; Rhodes & Tremewan, 1996; Rubenstein, Langlois, & Roggman, 2002). Other theories of attractiveness posit that faces that are symmetrical or that include large features (e.g., large eyes and lips for females) are the most attractive (e.g., Grammer & Thornhill, 1994; Perrett, May, & Yoshikawa, 1994); these theories, however, are based on studies with significant methodological shortcomings and, unlike averageneness, are neither necessary nor sufficient to explain attractiveness (Langlois et al., 1994; Rhodes & Tremewan, 1996; Rhodes et al., 1999; Rubenstein et al., 2002).
If attractive faces are prototypic, then they should be easier than unattractive faces to recognize and classify as being faces because they can be compared with a prototype quickly and efficiently (Johnston & Ellis, 1995). Using the same logic, attractive faces should be easier than unattractive faces to classify as being male or female. Indeed, O’Toole et al. (1998) found that facial attractiveness information facilitates adults’ sex classification in that adults rated faces that previously had been identified quickly as their respective sex as more attractive than faces more slowly identified as their respective sex.
Several lines of evidence suggest that attractive faces are treated as prototypes. Adults rate attractive faces as more typical in appearance and classify typical faces as faces more quickly than atypical or distinctive faces in face processing tasks (e.g., Light, Hollander, & Kayra-Stuart, 1981; Valentine & Bruce, 1986; Vokey & Read, 1992). Infants look longer at averaged female faces (composite faces made by averaging together 32 individual faces) than to unattractive female faces and can abstract a prototype female face from exposure to exemplar female faces (Rubenstein, Kalakanis, & Langlois, 1999). Furthermore, infants treat a prototype (averaged) female face as familiar even though they have not seen it prior to testing (de Haan, Johnson, Maurer, & Perrett, 2001; Rubenstein et al., 1999; Walton & Bower, 1993). Because averageness theory predicts that attractive faces are better examples of faces, a crucial test of the usefulness of averageness theory for social categorization would be to determine whether attractive, or prototypical, faces are more quickly and accurately classified as male or female than unattractive faces. If so, then the importance of averageness theory could be extended: Attractive faces are not only liked because they are better examples of face categories in general but also because they are better examples of male and female face categories specifically and therefore require less processing time in a sex classification task. The primary purpose of the following studies was to investigate the hypothesis that facial attractiveness facilitates quick and accurate identification of sex.
Facial information other than attractiveness also may be important for sex classification of faces. One type of facial information likely to facilitate sex classification is masculinity or femininity of facial appearance (Bruce, Ellis, Gibling, & Young, 1987; O’Toole et al., 1998). The influence of facial masculinity and femininity on sex classification is important to examine independent of the influence of facial attractiveness for several reasons. One, attractive male faces are not always perceived as being highly masculine, whereas attractive female faces generally are perceived as being highly feminine (O’Toole et al., 1998; Perrett et al., 1998; Rhodes, Hickford, & Jeffrey, 2000). Bronstad, Ramsey, and Langlois (2002) found that, with repeated random sampling of 147 female faces and 150 male faces, the correlation between attractiveness and femininity in female faces was relatively high and consistent (average r = .70), whereas the correlation between attractiveness and masculinity in males was more moderate and inconsistent (average r = .36). Also, Bronstad et al. determined that the correlation between rated attractiveness and rated femininity for female faces was always positive, but the correlation between rated attractiveness and rated masculinity for male faces was sometimes positive and sometimes negative. Taken together, these findings are similar to O’Toole et al.’s (1998) study in which they found a correlation of .88 for female attractiveness and femininity and a correlation of .23 for male attractiveness and masculinity. Given that masculinity and attractiveness may be capturing unique information in male faces, we predict that masculinity in male faces, but not femininity in female faces, may influence the speed and accuracy of sex classification independent of attractiveness.
Although O’Toole et al. (1998) previously found that faces classified more quickly as their respective sex tended to be more feminine and more attractive if female, and more masculine if male, the same group of raters judged both the attractiveness and masculinity/femininity of the face stimuli. Because having the same people provide ratings on correlated variables can produce a “halo effect” that exaggerates the ratings (e.g., Hoyt, 2000; Saal, Downey, & Lahey, 1980; Thorndike, 1920), our study addresses this potential source of bias by having different groups of people rate faces on either attractiveness or facial masculinity/femininity. In addition, O’Toole et al. did not select their facial stimuli to reflect significantly different levels of facial attractiveness and masculinity/femininity (rather, they investigated the relationship between speed of sex classification and attractiveness and masculinity/femininity after collecting the reaction time and rating data). Our study remedies this weakness by pre-selecting face stimuli from two levels of facial attractiveness and two levels of facial masculinity/femininity, thus allowing comparison of effects and interactions for low and high levels of attractiveness and masculinity/femininity.
In the following studies, we made separate predictions for how the cues of attractiveness and masculinity/femininity would facilitate adults’ and children’s sex classification of female and male faces. For female faces, we predicted that high attractive, high feminine faces should be identified the most rapidly and accurately as female by both children and adults. For male faces, we predicted that high attractive, high masculine male faces also should be identified the most rapidly and accurately as male by adults but not by children. Instead, because young children typically have less experience with adult male versus adult female and children’s faces (e.g., Harrison & Magill-Evans, 1996; Russell & Radojevic, 1992; Wille, 1995), they may have a general facial prototype that is more female-like in representation, or they may have a better-formed female versus male facial prototype if they have separate prototypes for female and male faces. Although there is insufficient evidence to determine empirically whether young children have a general prototype for all types of faces or separate prototypes for the two sexes, either possibility suggests children may perceive attractive female faces as prototypic, but may not yet perceive attractive male faces as prototypic. Thus, we predicted that masculinity cues might have equal or greater importance than attractiveness for children’s sex classification of male faces.
Experiment 1
To investigate whether the facial cues of attractiveness and masculinity/femininity interactively or independently influence adults’ identification of sex, we asked adults to perform a reaction time (RT) task in which they classified, as quickly as possible, the sex of male and female faces.
Method
Participants
We recruited 33 adults (17 women, mean age = 25.5 years) from the Austin community, who volunteered to participate in the study. This sample included participants of Caucasian (79%), Hispanic (15%), and Asian-Pacific Islander (3%) descent. The remaining participants (3%) were either mixed race or “other.” The data from five adults were excluded from analyses for the following reasons: experimenter error (2), voice activation errors on multiple trials (i.e., not speaking loudly enough; 2), and missing data (i.e., having two incorrect responses for a particular type of face; 1). The final sample included data from 28 adults (14 women) ranging in age from 18 to 39 years (M= 26.43 years, SD = 5.77 years).
Stimuli
We randomly chose 200 images of adult male (N = 100) and female (N = 100) Caucasian faces from an existing database of high and low attractive images of faces of undergraduate students. Within each sex category of faces, half the faces were high attractive and half the faces were low attractive. The faces had been rated previously for attractiveness by at least 40 undergraduates (20 female) on a 5-point Likert scale (1 = very unattractive, 5 = very attractive); the ratings were highly reliable (alphas = .90 or higher). The selected faces represented the range of attractiveness found within the population of healthy, normal, young adult faces; none were movie stars and none were deformed. An independent group of at least 40 undergraduates (20 female) rated these faces for masculinity or femininity on a 5-point Likert scale (1 = not very feminine, 5 = very feminine for female faces; 1 = not very masculine, 5 = very masculine for male faces). The masculinity and femininity ratings were highly reliable (alpha = .97) and correlated positively with the attractiveness ratings (r = .40 for male faces, r = .80 for female faces). Although male faces were rated for masculinity only and female faces were rated for femininity only, we refer to these variables together as masculinity/femininity for ease of communication; henceforth, when we refer to the variable of masculinity/femininity, we mean masculinity for male faces and femininity for female faces.
From the initial pool of 200 facial images, we selected 12 for each sex including three each of the following face types for both female and male faces: low attractive, low masculine/feminine; low attractive, high masculine/feminine; high attractive, low masculine/feminine; and high attractive, high masculine/feminine. Because of the high positive correlation between attractiveness and femininity for the female faces, we were unable to choose more than a few examples from certain face types (e.g., high attractive, low feminine female faces). Therefore, we limited the number of examples of all face types to three examples each for a total of 12 faces of each sex. Within face types, examples were chosen based on having the highest or lowest attractiveness and masculinity/femininity scores with the constraint that characteristics such as hair color were equally represented across categories.
To determine whether mean group attractiveness scores were significantly different across attractiveness and masculinity/femininity levels but not within attractiveness and masculinity/femininity, we conducted several t-test comparisons. The ratings of the faces in the low and high attractive conditions were significantly different from one another, p-values < .0001 for both the female and male faces. There were also significant differences between the two levels of femininity within each of the two levels of attractiveness for the female faces and between the two levels of masculinity within each of the two levels of attractiveness for the male faces (all p-values < .05). For the female faces, the ratings for the six low attractive faces ranged from 1.69 to 1.84 (M = 1.81) and the ratings for the six high attractive faces ranged from 3.17 to 4.19 (M = 3.50). Also, the ratings for the six low feminine faces ranged from 2.32 to 3.52 (M = 2.80), and the ratings for the six high feminine faces ranged from 3.66 to 4.55 (M = 4.13). For the male faces, the ratings for the six low attractive faces ranged from 1.18 to 1.55 (M = 1.46), and the ratings for the six high attractive faces ranged from 3.09 to 3.54 (M = 3.31). Additionally, the ratings for the low masculine faces ranged from 1.80 to 2.87 (M = 2.54), and the ratings for the high masculine faces ranged from 4.07 to 4.65 (M = 4.31).
To make the task manageable for younger participants (see Experiment 2), all participants viewed only two of the three examples of every combination. We also selected six children’s faces (three girls, three boys) of similar attractiveness to be used in a practice portion of the study. Children’s faces were selected in order to introduce participants to the task without sensitizing them to variations in facial attractiveness or masculinity/femininity. All faces were standardized for size, color, brightness, and contrast using Adobe Photoshop™. Additionally, all faces had neutral expressions, and all clothing cues were masked.
Procedure
Participants sat in front of a computer screen, which displayed the facial images. The images appeared one at a time on the computer screen for a maximum of 3000 milliseconds (ms) via Superlab™. The on-screen size of each facial image was 700 by 700 pixels, just slightly smaller than life-size. To make our task more manageable for our child participants (Experiment 2), we employed a verbal response methodology rather than a manual response methodology. We reasoned that a verbal task, in which participants responded with one of two words, would be less confusing and time-consuming to children than a manual search task, in which participants needed to look for and press one of two computer keys (which would be difficult to clearly label for children who cannot yet read). In accordance with our verbal methodology, we asked the adults to respond to the faces verbally by saying either “boy” or “girl” as soon as they knew the sex of the face. We chose the words “boy” and “girl” because pilot testing demonstrated that they were both easy to pronounce and familiar to young children. A microphone centered at the bottom of the computer screen recorded the onset of each verbal response and was set at a low threshold in order to register low volume responses. Once participants made a response or reached the maximum time allotted (3000 ms) for that trial, the face disappeared. To control for any visual “after effects” and to give participants a brief break between each face, a white screen appeared for 2000 ms and then a black dot, centered on a white screen, appeared for 750 ms prior to the presentation of each face.
Before viewing the adult stimulus faces, the participants first viewed and responded to the six children’s faces to provide practice with the general method of the study. Once they understood the task, they viewed the series of adult faces in two blocks so that a break in presentation separating each of the blocks allowed a brief rest period. Each participant viewed and responded to 16 of 24 possible faces in one of 12 random orders, and the orders were counterbalanced across participants so that all faces were shown an equal number of times overall.
Results and Discussion
Reaction Times
To analyze reaction times for identifying faces as female or male, we first averaged the reaction times (in ms) for each of the eight face types (see Table 1). Adult participants made few errors, but if they made an error to one example of a face type (e.g., the first low attractive, low masculine male face), then only the reaction time to the other face of that type (e.g., the second low attractive, low masculine male face) was used for data analysis. Errors included either misidentifying a face (e.g., identifying a female face as a “boy”) or making a voice activation error (e.g., not talking loud enough during a stimulus presentation).
Table 1.
Adults’ Reaction Times in MS to All Face Types
| Female faces | Male faces | |
|---|---|---|
| High attractive, high | 614.43 (M) | 601.64 (M) |
| masculine/feminine faces | 143.16 (SD) | 134.56 (SD) |
| High attractive, low | 598.48 (M) | 748.66 (M) |
| masculine/feminine faces | 152.86 (SD) | 276.95 (SD) |
| Low attractive, high | 653.27 (M) | 628.84 (M) |
| masculine/feminine faces | 135.79 (SD) | 138.21 (SD) |
| Low attractive, low | 672.11 (M) | 835.79 (M) |
| masculine/feminine faces | 157.88 (SD) | 319.18 (SD) |
To determine the independent versus interactive effects of facial attractiveness and masculinity/femininity on how quickly adults identified sex, we initially analyzed the data with a 2 (participant sex) × 2 (facial attractiveness) × 2 (facial masculinity/femininity) × 2 (sex of face) repeated measures ANOVA. The between-participants variable was participant sex; within-participants variables were the facial attractiveness, facial masculinity/femininity, and sex of face. Because this analysis showed no effect of participant sex, we collapsed the data across this variable and analyzed the three within-participants variables.
Results showed a main effect of facial attractiveness that was not qualified by any higher order interaction: As predicted, adults identified the sex of the high attractive faces (M = 640.80, SD = 151.29) significantly faster than the low attractive faces (M = 697.50, SD = 167.27), F (1, 27) = 7.22, p < .05. Results also demonstrated a significant two-way interaction between facial masculinity/femininity and sex of face, F (1, 27) = 35.30, p < .001. In interpreting this interaction, we controlled for Type 1 error across multiple comparisons by using Bonferroni corrections. Paired comparison t-tests showed that adults identified the sex of the high masculine male faces (M = 615.24, SD = 131.10) significantly more quickly than the low masculine male faces (M = 792.22, SD = 249.22), t (27) = 5.89, p < .001; adults, however, evidenced no difference in how quickly they identified the sex of the high feminine female faces (M = 633.85, SD = 128.20) versus the low feminine female faces (M = 635.30, SD = 137.35), t (27) = .138, p = n.s. (see Figure 1). There were no other significant interactions.
Figure 1.
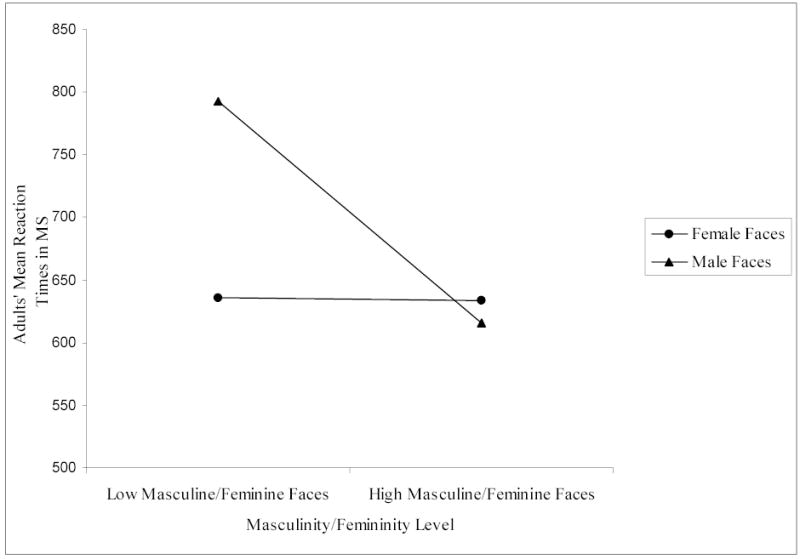
Adults’ mean reaction times in ms to high and low masculine/feminine female and male faces.
High levels of facial attractiveness for all faces and facial masculinity for male faces facilitated the speed of adults’ classification of sex in Experiment 1. These results were similar to those found by O’Toole et al. (1998) except that high attractive, high feminine female faces were not identified more quickly than their low attractive, low feminine counterparts in our study. Instead, high attractive faces of both sexes were identified as their respective sex faster than low attractive faces regardless of their facial masculinity/femininity. Independent of facial attractiveness, level of facial masculinity/femininity facilitated rapid sex classification of male, but not female, faces. By pre-selecting stimuli that ranged in attractiveness and masculinity/femininity, our results more precisely demonstrated that the cue of attractiveness (or averageness) aids in adults’ identification of sex independent of both masculinity/femininity and whether a face is male or female.
Errors in Sex Classification
Although adult participants made some incorrect responses (3% of all responses were errors), the total number of incorrect responses (N = 14) was too small to statistically analyze. Descriptively, though, adults made errors most often on low attractive, low masculine male faces (12). There was also one error each for high attractive, low masculine male faces and low attractive, low feminine female faces. Hence, if adults made mistakes in classifying sex, they were most likely to err on male versus female faces and especially on male faces lower in both attractiveness and masculinity. This finding fits with our prediction that participants should be less accurate in identifying the sex of low attractive and low masculine/feminine faces and suggests that low attractive, low masculine male faces were sometimes perceived as female.
Experiment 2
After determining that facial attractiveness facilitates sex classification independent of facial masculinity/femininity for adults, we wanted to assess if and how the cues of facial attractiveness and facial masculinity/femininity affect sex classifications for children. Similar to our results with adults, we expected that attractiveness (or averageness) should be more important than femininity for identifying the sex of female faces. Conversely, because children may have a female-like general facial prototype or better-formed prototypes for adult female versus adult male faces (if they have sex-specific prototypes), we expected that masculinity information might be more important than attractiveness for classifying the sex of male faces. We tested 4–5-year-olds because, although previous research has shown that children as young as 5 years of age more rapidly identify prototypical faces than distinctive faces as being faces (Johnston & Ellis, 1995), no one to our knowledge has tested this age group of children using a RT task for the identification of sex of face.
Method
Participants
We recruited 245 four- and five-year-old children (124 girls, mean age = 4.8 years), including children of Caucasian (76%), Hispanic (12%), Asian-Pacific Islander (2%), and African American (2%) descent. The remaining participants (8%) were either mixed race or “other.” Published birth announcements in a local newspaper provided the initial source of participant names. The names provided by birth announcements were entered into a database; we contacted parents of all potential 4–5-year-old participants with a letter explaining the experiment and followed up with a telephone call to schedule an appointment.
The data from 102 children were excluded from data analyses for the following reasons: participant refusal to either begin or complete the task (40); voice activation errors on multiple trials (i.e., not talking loud enough, talking or laughing at the start of a response, using responses other than “boy” or “girl,” and responding before the photograph fully appeared on screen; 36); missing data (22); experimenter error (3); and equipment error (1).
The final sample included data from 143 children (77 girls) ranging in age from 3.99 to 5.72 years (M = 4.86 years, SD = .51 years). Although similar in age to the original sample, the final sample was significantly different in race and sex from the original sample in that it had proportionally fewer Caucasian children and boys than the original sample.
Stimuli and Procedure
The stimuli and procedure were identical to that of Experiment 1 except that if children had difficulty with the practice portion of the task, they practiced with the children’s faces until they demonstrated comprehension of the procedure. Also, as with the adults, we presented the faces in two blocks for the children closer to 5 years of age; for children closer to 4 years of age, however, we presented the faces in four blocks because pilot testing suggested the younger children needed more breaks to successfully complete the task.
Results and Discussion
Reaction Times
As with the adult data in Experiment 1, we first averaged the reaction times (in ms) for each of the eight types of faces in order to analyze children’s reaction times for identifying faces as female or male (see Table 2). Again, if children either misidentified a face or made a voice activation error on one example of a face type, only the reaction time to the other example of that face type was included in the data analysis. Because data can be correlated within a participant in a repeated measures design (Hays, 1994), we could not find an appropriate method for analyzing partially missing data, and, therefore, we could not analyze data for any children who made voice activation errors or who misidentified a face during both examples of a face type (e.g., both low attractive, low masculine male faces).
Table 2.
Children’s Reaction Times in MS to All Face Types
| Female faces | Male faces | |
|---|---|---|
| High attractive, high | 909.92 (M) | 884.76 (M) |
| masculine/feminine faces | 259.16 (SD) | 297.61 (SD) |
| High attractive, low | 906.24 (M) | 943.49 (M) |
| masculine/feminine faces | 265.87 (SD) | 342.08 (SD) |
| Low attractive, high | 1103.79 (M) | 915.70 (M) |
| masculine/feminine faces | 375.72 (SD) | 289.77 (SD) |
| Low attractive, low | 1032.55 (M) | 994.34 (M) |
| masculine/feminine faces | 396.71 (SD) | 334.25 (SD) |
To determine the effects of facial attractiveness and masculinity/femininity on how quickly children identified sex, we first analyzed the data with a 2 (participant sex) × 2 (facial attractiveness) × 2 (facial masculinity/femininity) × 2 (sex of face) repeated measures ANOVA. The between-participants variable was participant sex; within-participants variables were facial attractiveness, facial masculinity/femininity, and sex of face. In addition to significant and predicted main effects and two-way interactions, this analysis resulted in a single significant three-way interaction between participant sex, facial masculinity/femininity, and sex of face, F (1, 141) = 5.13, p < .05. We did not investigate this interaction further for the following reasons: we found no effect of participant sex in Experiment 1; there were no main effects or two-way interactions involving participant sex; there is no empirical history of participant sex effects in sex classification studies with children; and there was no theoretical reason to predict such a higher-order interaction. We therefore collapsed data across the variable of participant sex to analyze the data with a 2 (facial attractiveness) × 2 (facial masculinity/femininity) × 2 (sex of face) repeated measures ANOVA.
Results showed significant two-way interactions between facial attractiveness and sex of face, F (1, 142) = 15.32, p < .001, and facial masculinity/femininity and sex of face, F (1, 142) = 9.69, p < .005. We conducted paired samples t-tests to interpret the significant interactions. For all comparisons, we controlled for Type 1 error across multiple comparisons using Bonferroni corrections. As predicted, attractiveness was important for children’s sex classification of female faces, but not male faces: Children identified the sex of the high attractive female faces (M = 908.08, SD = 206.98) significantly faster than the low attractive female faces (M = 1068.17, SD = 296.82), t (142) = 7.05, p < .001, whereas there was no difference in children’s speed of sex classification for the high attractive male faces (M = 914.12, SD = 245.85) versus the low attractive male faces (M = 955.02, SD = 244.71), t (142) = 1.74, n.s. (see Figure 2). Also as predicted, masculinity was important for children’s sex classification of male faces: Children identified the sex of the high masculine male faces (M = 900.23, SD = 230.52) significantly faster than the low masculine male faces (M = 968.91, SD = 259.06), t (142) = 2.93, p < .01, whereas there was no difference in their speed of sex classification of the high feminine female faces (M = 1006.86, SD = 263.81) versus the low feminine female faces (M = 969.39, SD = 254.83), t (142) = 1.58, n.s. (see Figure 3). No other interactions were significant.
Figure 2.
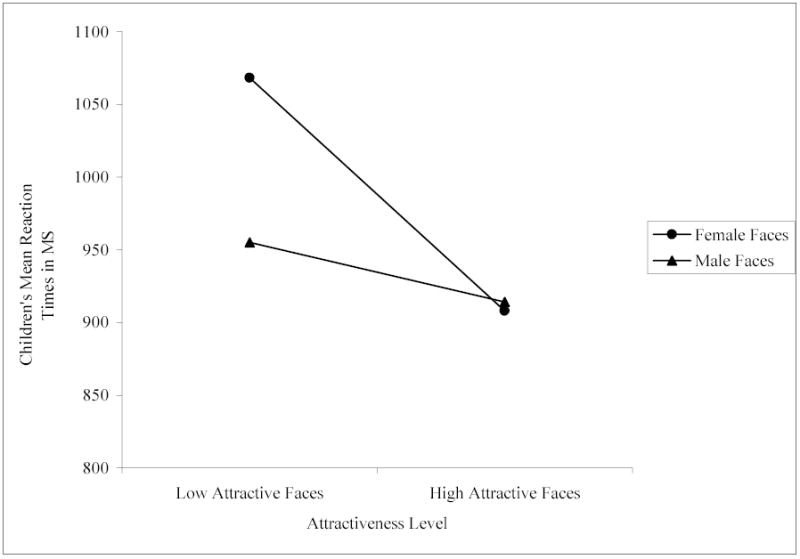
Children’s mean reaction times in ms to high and low attractive female and male faces.
Figure 3.
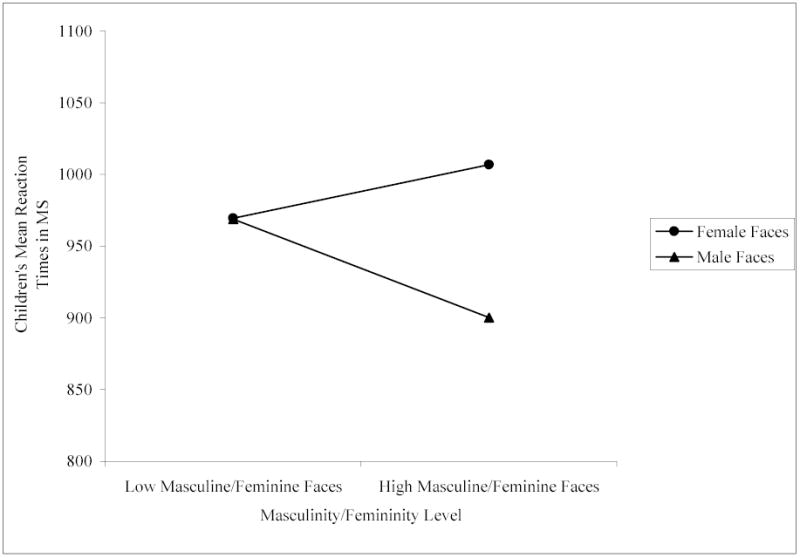
Children’s mean reaction times in ms to high and low masculine/feminine female and male faces.
These results indicate that children as young as preschool-age make use of attractiveness cues when classifying the sex of female faces and make use of masculinity cues when classifying the sex of male faces. As with the adults in Experiment 1, attractiveness cues facilitated children’s identification of sex independent of femininity cues for female faces: Children classified the sex of high attractive female faces faster than low attractive female faces. Unlike the adults, however, attractiveness cues were not important for the classification of male faces: Children identified the sex of high and low attractive male faces with equal speed. Like the adults and as predicted, however, high masculinity appeared to facilitate children’s identification of males because children classified the sex of high masculine male faces faster than low masculine male faces. Also like the adults, they classified the sex of high and low feminine faces with equal speed to one another.
Errors in Sex Classification
Children’s error rate was fairly low (17%) but frequent enough to analyze statistically (see Figure 4 for total number of children’s errors to all face types). To assess whether any of the stimuli variables (sex of face, facial attractiveness, and facial masculinity/femininity) predicted the accuracy of sex classification, we first determined how many errors each child made for each of the eight types of faces. Errors were defined as either no response to a face or an incorrect response to a face (e.g., identifying a female face as a “boy”). Errors made because of equipment problems or performing the task incorrectly were not included as errors in sex classification. Because of a priori rules for the analysis of missing data, children could either make no errors or just one error for a face type; therefore, for each of the eight face types, children received a score of 0 or 1 for the number of errors made on that face type.
Figure 4.
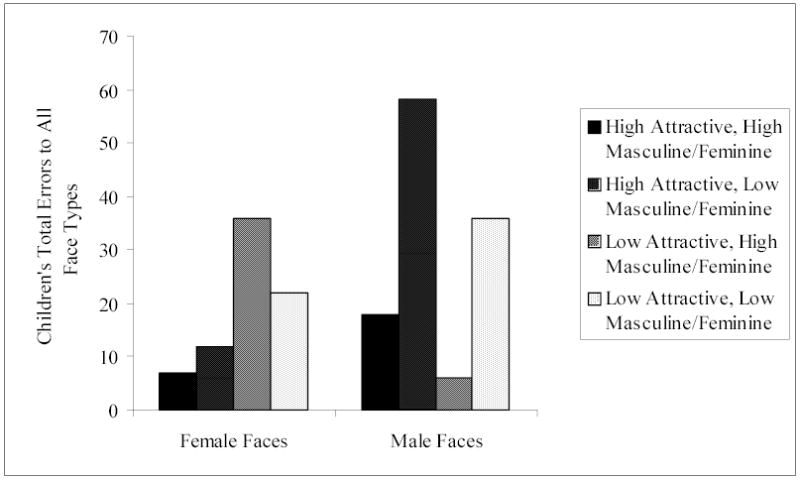
Children’s total number of errors to all face types.
Because the data were binary and because errors could have been correlated within participants due to the repeated measures design, the most appropriate way to model the error data and to assess the effects of predictor variables was a generalized linear modeling procedure. This model applies generalized estimating equations (GEEs) to determine both main effects and interaction effects of predictor variables on categorical responses (see Liang & Zeger, 1986 for an explanation of GEEs).
All results of the GEEs analysis are reported as Wald statistics (Wald, 1943). After testing the most restricted model (one in which all three predictor variables are included) against less restrictive models (models in which at least one predictor variable was not included), chi-square statistics showed that the most restrictive model accounted for the most variance in error judgments, χ2 (1140, N = 143) = 1177.04. This model resulted in an overall three-way interaction between the variables of sex of face, attractiveness, and masculinity/femininity, χ2 (1, N = 143) = 5.75, p < .05. To interpret this interaction, we conducted simple effects analyses between sex of face and attractiveness, and sex of face and masculinity/femininity. For all comparisons, we controlled for Type 1 error across multiple comparisons using Bonferroni corrections.
The simple effects analyses resulted in significant two-way interactions between sex of face and attractiveness, χ2 (1, N = 143) = 32.52, p < .0001, and between sex of face and masculinity/femininity, χ2 (1, N = 143) = 31.24, p < .001. Because simple effects do not allow for an interpretation of the direction of effects (see Winer, Brown, & Michels, 1991), we conducted further simple effects analyses within sex of face by attractiveness and within sex of face by masculinity/femininity.
In the sex of face by attractiveness simple effects analysis, we collapsed the variable of masculinity/femininity across attractiveness levels for both female and male faces. These results showed that, for female faces, the likelihood of a child making a sex classification error increased as the attractiveness of the face decreased, β = 1.22, χ2 (1, N = 143) = 19.71, p < .0001. In contrast, for male faces, the likelihood of a child making a sex classification error increased as the attractiveness of the face increased, β = −0.77, χ2 (1, N = 143) = 12.82, p < .001.
In the sex of face by masculinity/femininity simple effects analysis, we collapsed the variable of attractiveness across masculinity/femininity levels for both female and male faces. Results demonstrated that, for male faces, the likelihood of a child making a sex classification error increased as the masculinity of the face decreased, β = 1.72, χ2 (1, N = 143) = 47.00, p < .0001. Level of femininity, however, did not affect the likelihood of errors in children’s sex classification of female faces, β = −0.24, χ2 (1, N = 143) = .95, n.s.
The results of children’s errors in sex classification correspond to the results of children’s reaction times in identifying sex of face. Specifically, children were both slower and more likely to make an error in identifying the sex of a low attractive female face as compared to a high attractive female face. Additionally, level of femininity did not affect children’s errors in sex classification just as it did not affect their speed in reaction times. For male faces, children were both faster and less likely to err on a high versus a low masculine male face. Interestingly, children were more likely to err on high relative to low attractive male faces. Although this result does not explain why children identified high and low attractive male faces with equal speed, it suggests why high attractiveness did not facilitate the speed of sex classification more than low attractiveness for male faces: Because they were more apt to err in their classification of high versus low attractive male faces, children likely required more processing time than would be expected to successfully identify the sex of high versus low attractive male faces.
General Discussion
Previous literature has suggested that facial configuration as a whole is more informative than individual facial features such as mouth or eye shape in helping people to correctly identify sex (e.g., Bruce et al., 1993; Campanella et al., 2001; Yamaguchi et al., 1995), yet no study until ours has examined if an attractive or masculine/feminine facial configuration facilitates the task of sex classification for both adults and children. The results from our two experiments demonstrate that facial attractiveness helps adults and children to identify sex, although the importance of facial attractiveness for male faces depends on whether the perceiver is an adult or a young child. Whereas facial attractiveness facilitated adults’ sex classification of both female and male faces, attractiveness facilitated children’s sex classification of female faces only. In contrast, facial masculinity facilitated both children’s and adults’ sex classification decisions similarly. Our discussion explores these interesting differences in the sex classification of female versus male faces as well as speculates about the implications of our findings for Langlois & Roggman’s (1990) averageness theory of attractiveness preferences.
Sex Classification of Female Faces
Our prediction that high attractiveness combined with high femininity would lead to the fastest and most accurate sex classification of female faces was not supported because the variable of facial attractiveness did not interact with the variable of facial femininity for either adults or children. Instead, for both adults and children, only high attractiveness facilitated sex classification of female faces regardless of high femininity. These results extend O’Toole et al.’s (1998) findings by showing that, although attractiveness generally correlates with femininity for female faces, high attractiveness appears to be a more influential cue than high femininity in facilitating accurate sex classification when the two variables are manipulated independently. Thus, people may prefer very attractive female faces because such faces are prototypic, good examples of female faces, and easier to process.
Sex Classification of Male Faces
In our experiments, children relied more on masculinity than attractiveness cues when deciding the sex of a male face, whereas adults relied on both attractiveness and masculinity cues, though independently of one another, in the same task. All participants’ faster and more accurate identification of high masculine relative to low masculine male faces fits with previous findings with adults that masculinity is an informative cue in the sex classification of male faces (Bruce et al., 1987; O’Toole et al., 1998). Why is masculinity useful for identifying the sex of male faces, particularly for young children? Children’s greater experiences with adult female and children’s faces versus adult male faces may solidify the prototypicality of attractive female faces; therefore, children may use masculinity cues to help identify adult faces that are dissimilar to either their general female-like face prototype or their well-formed sex-specific prototypes for female faces. Our data do not allow us to determine whether children are using general or sex-specific prototypes when making sex classification decisions, but either possibility fits with our predictions and results.
If attractive female faces are more prototypical than attractive male faces to children, then attractive female faces would fall nearer to the center of their geometric, multidimensional “face space” than attractive male faces. Face space is a metaphorical framework for understanding the mental representation of faces in which faces are stored within locations in a multidimensional space, and the dimensions of the space correspond to ways in which faces can vary and be encoded (Valentine, 1991). If attractive female faces are at the center of face space, then less attractive and less female-like faces should be farther away from the center of face space. Because low attractive, high masculine male faces are conceivably the least “female-like” and therefore the farthest faces away from the center of face space, these faces should be easily identified as being “not female.” Support for this hypothesis comes from our finding that children were least likely to make mistakes on low attractive, high masculine male faces than on any other type of face.
The Importance of Sex Classification
Prior research has found that infants and adults evidence visual preferences for attractive faces and perceive them as more face-like, perhaps because they are more average (in facial configuration) or prototypical (Langlois et al., 1987; Langlois & Roggman, 1990; Light et al., 1981; Rubenstein et al., 1999). In this study, we found that adults identify the sex of high attractive female and male faces faster than their low attractive counterparts and that children identify the sex of high attractive female faces faster and more accurately than their low attractive counterparts. That high attractive faces are identified as their respective sex faster than low attractive faces provides an additional explanation as to why attractive faces are appealing to young children and adults. Specifically, more attractive faces are not just average in facial appearance and therefore more prototypical and face-like but are also prototypical of sex appearance and therefore more “female-like” and “male-like.”
Most participants in our task correctly identified the sex of the faces within the allotted time frame, suggesting that sex classification is an automated task. Indeed, identifying sex is a crucial social action that must occur almost instantaneously for social interaction purposes. Our results show that an especially attractive appearance for female targets (and male targets when adults are the perceivers) and an especially masculine appearance for male targets facilitate sex classification, which likely has useful social consequences. On the other hand, rapid sex categorization in modern society also could be disadvantageous for targets in that categorization often precedes stereotype formation. Specifically, faces that are easily identified as male or female may be perceived as being more sex-typed than other male and female faces. Indeed, research shows that both children and adults perceive females with high attractive faces as being more likely to have feminine traits and as being more likely to engage in feminine activities and occupations than females with low attractive faces (Gillen, 1981; Hoss & Langlois, 2003).
Directions for Future Research
Although our findings are in accord with those of O’Toole et al. (1998) who found that adults quickly identify the sex of faces that are highly attractive and the sex of male faces that are highly masculine in appearance, we were able to draw more precise conclusions about how independent levels of facial attractiveness and masculinity/femininity affect the speed and accuracy of sex classification. By independently varying levels of both attractiveness and masculinity/femininity in our experiments, we were able to determine whether the two types of cues interacted or made independent contributions to the sex classification of faces. One limitation to our studies is that, because of the attentional limitations of the children we tested, we showed only 24 adult faces as stimuli, which may limit the generalization of our findings to other stimuli sets of different races or ages. Other stimuli should be employed in similar studies to bolster our findings. Child faces as stimuli could result in particularly interesting findings in that sex-typical appearance cues (e.g., high masculinity for male faces) are less prominent in prepubescent children’s faces than in adults’ faces (Enlow, 1982), which would suggest that attractiveness might be more important than masculinity/femininity in the sex classification of young children’s faces. Recent research, though, has shown that adults detect a range of facial masculinity and femininity in children as young as 3-years-old and even make sex-typed attributions to children based on differences in facial masculinity/femininity when attractiveness is held constant (Rogers & Ritter, 2002). Because it is unclear how attractiveness and masculinity/femininity would affect the sex classification of children’s faces, studies employing stimulus faces of varying ages are needed.
Finally, although our pilot work for this study showed that 4-years-old is the youngest age group that can reliably perform the sex classification task we designed, future research should assess how facial cues such as attractiveness and masculinity/femininity aid children younger than 4-years-old in sex classification using other age-appropriate methodology. Sex categorization of target faces is an important social task that is believed to develop in infancy; existing research on the development of sex categorization in infancy, however, has used facial stimuli that are highly attractive and sex-typical in appearance rather than facial stimuli that range in attractiveness and typicality (e.g., Leinbach & Fagot, 1993). Given our findings that attractiveness and sex-typical cues facilitate faster and more accurate sex categorization for children and adults, the findings from infant sex categorization studies are limited because they do not clearly show that infants can easily categorize the sex of faces that are low attractive or low masculine/feminine in facial appearance. Indeed, recent research suggests that infants have difficulty categorizing sex at 11 months of age and younger if the faces are atypical in appearance (Newell & Strauss, 2002; Newell, Strauss, & Best, 2003).
Conclusions
In support of our predictions, we found that a highly attractive face facilitates quick and accurate sex classification but that facial attractiveness information is more useful for identifying female faces than male faces during childhood and equally useful for identifying both female and male faces during adulthood. Perhaps attractiveness becomes a more important cue for the sex classification of male faces sometime during the early adolescent years when identifying another’s sex might be especially important for social interaction and dating. Additionally, we found that masculinity in male faces, but not femininity in female faces, independently aids both children and adults in sex classification tasks; moreover, the importance of masculinity cues does not appear to differ between early childhood and adulthood. These results taken together suggest that facial attractiveness and facial masculinity information both play an important, yet independent, role in facilitating even young children’s quick and accurate sex classification.
Footnotes
Rebecca A. Hoss is now at the Department of Psychology, College of Saint Mary, Omaha, Nebraska.
Jennifer L. Ramsey is now at the Department of Psychology, University of Nevada at Las Vegas.
References
- Bronstad, P.M., Ramsey, J.L., & Langlois, J.H. (2002, June). Sample size explains discrepancies in facial attractiveness research: Masculine male faces are more attractive (formerly Femininity = attractiveness but masculinity and attractiveness merely share variance). Poster session presented at 14th annual meeting of American Psychological Society, New Orleans, LA.
- Brown E, Perrett DI. What gives a face its gender? Perception. 1993;22:829–840. doi: 10.1068/p220829. [DOI] [PubMed] [Google Scholar]
- Bruce V, Burton AM, Hanna E, Healey P, Mason O, Coombes A, Fright R, Linney A. Sex discrimination: How do we tell the difference between male and female faces? Perception. 1993;22:131–152. doi: 10.1068/p220131. [DOI] [PubMed] [Google Scholar]
- Bruce V, Ellis H, Gibling F, Young A. Parallel processing of the sex and familiarity of faces. Canadian Journal of Psychology. 1987;41:510–520. doi: 10.1037/h0084165. [DOI] [PubMed] [Google Scholar]
- Burton AM, Bruce V, Dench N. What’s the difference between men and women? Evidence from facial measurement. Perception. 1993;22:153–176. doi: 10.1068/p220153. [DOI] [PubMed] [Google Scholar]
- Campanella S, Chrysochoos A, Bruyer R. Categorical perception of facial gender information: Behavioural evidence and the face-space metaphor. Visual Cognition. 2001;8:237–262. [Google Scholar]
- de Haan M, Johnson MH, Maurer D, Perrett DI. Recognition of individual faces and average face prototypes by 1- and 3-month-old infants. Cognitive Development. 2001;16:659–678. [Google Scholar]
- Enlow, D. (1982). Handbook of facial growth. Philadelphia: W. H. Saunders.
- Gillen B. Physical attractiveness: A determinant of two types of goodness. Personality and Social Psychology Bulletin. 1981;7:277–281. [Google Scholar]
- Grammer K, Thornhill R. Human (homo sapiens) facial attractiveness and sexual selection: The role of symmetry and averageness. Journal of Comparative Psychology. 1994;108:233–242. doi: 10.1037/0735-7036.108.3.233. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, Magill-Evans J. Mother and father interactions over the first year with term and preterm infants. Research in Nursing & Health. 1996;19:451–459. doi: 10.1002/(SICI)1098-240X(199612)19:6<451::AID-NUR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hays, W. L. (1994) Statistics (5th ed.). Fort Worth: Harcourt Brace.
- Hoss, R. A., & Langlois, J. H. (2003, May). Children’s attributions of gender stereotypes to faces varying in attractiveness. Poster session presented at the 15th annual meeting of American Psychological Society, Atlanta, GA.
- Hoyt WT. Rater bias in psychological research: When is it a problem and what can we do about it? Psychological Methods. 2000;5:64–86. doi: 10.1037/1082-989x.5.1.64. [DOI] [PubMed] [Google Scholar]
- Intons-Peterson, M. (1988). Children’s concepts of gender. Norwood, NJ: Alex.
- Johnston RA, Ellis HD. Age effects in the processing of typical and distinctive faces. The Quarterly Journal of Experimental Psychology. 1995;48A:447–465. doi: 10.1080/14640749508401399. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Ritter JM, Roggman LA, Vaughn LS. Facial diversity and infant preferences for attractive faces. Developmental Psychology. 1991;27:79–84. doi: 10.1037//0012-1649.35.3.848. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Roggman LA. Attractive faces are only average. Psychological Science. 1990;1:115–121. [Google Scholar]
- Langlois JH, Roggman LA, Casey RJ, Ritter JM, Rieser-Danner LA, Jenkins VY. Infant preferences for attractive faces: Rudiments of a stereotype? Developmental Psychology. 1987;23:363–369. [Google Scholar]
- Langlois JH, Roggman LA, Musselman L. What is average and what is not average about attractive faces? Psychological Science. 1994;5:214–220. [Google Scholar]
- Leinbach MD, Fagot BI. Categorical habituation to male and female faces: Sex schematic processing in infancy. Infant Behavior and Development. 1993;16:317–332. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Light LL, Hollander S, Kayra-Stuart F. Why attractive people are harder to remember. Personality and Social Psychology Bulletin. 1981;7:269–276. [Google Scholar]
- Newell, L.C., & Strauss, M.S. (2002, April). Infants’ ability to discriminate gender using internal facial features. Poster session presented at the biennial meeting of the International Conference on Infant Studies, Toronto, Ontario.
- Newell, L.C., Strauss, M.S., & Best, C.A. (2003, April). Gender categorization during infancy: The effects of typicality and hair. Poster session presented at the biennial meeting of the Society for Research in Child Development, Tampa, FL.
- O’Toole AJ, Deffenbacher KA, Valentin D, McKee K, Huff D, Abdi H. The perception of face gender: The role of stimulus structure in recognition and classification. Memory & Cognition. 1998;26:146–160. doi: 10.3758/bf03211378. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, Henzi SP, castles DL, Akamatsu S. Effects of sexual dimorphism on facial attractiveness. Nature. 1998;394:884–887. doi: 10.1038/29772. [DOI] [PubMed] [Google Scholar]
- Perrett DI, May KA, Yoshikawa S. Facial shape and judgments of female attractiveness. Nature. 1994;368:239–242. doi: 10.1038/368239a0. [DOI] [PubMed] [Google Scholar]
- Ramsey, J. L. (2003). Infant attention to male faces. Doctoral dissertation: The University of Texas at Austin.
- Rhodes G, Hickford C, Jeffrey L. Sex-typicality and attractiveness: Are supermale and superfemale faces super attractive? British Journal of Psychology. 2000;91:125–140. doi: 10.1348/000712600161718. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Watson TL, Clifford CWG, Nakayama K. Fitting the mind to the world: Face adaptation and attractiveness aftereffects. Psychological Science. 2003;14:558–566. doi: 10.1046/j.0956-7976.2003.psci_1465.x. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Sumich A, Byatt G. Are average facial configurations attractive only because of their symmetry? Psychological Science. 1999;10:52–58. [Google Scholar]
- Rhodes G, Tremewan T. Averageness, exaggeration, and facial attractiveness. Psychological Science. 1996;7:105–110. [Google Scholar]
- Roberts T, Bruce V. Feature saliency in judging the sex and familiarity of faces. Perception. 1988;17:475–481. doi: 10.1068/p170475. [DOI] [PubMed] [Google Scholar]
- Rogers CM, Ritter JM. The power of perception: Children’s appearance as a factor in adults’ predictions of gender-typical behavior. Social Development. 2002;11:409–426. [Google Scholar]
- Rubenstein AJ, Kalakanis L, Langlois JH. Infant preferences for attractive faces: A cognitive explanation. Developmental Psychology. 1999;35:848–855. doi: 10.1037//0012-1649.35.3.848. [DOI] [PubMed] [Google Scholar]
- Rubenstein, A. J., Langlois, J. H., & Roggman, L. A. (2002). What makes a face attractive and why: The role of averageness in defining facial beauty. In G. Rhodes & L. A. Zebrowitz, (Eds), Facial attractiveness: Evolutionary, cognitive, and social perspectives. Advances in visual cognition, vol. 1 (pp. 1–33).
- Russell G, Radojevic M. The changing role of fathers? Current understandings and future directions for research and practice. Infant Mental Health Journal. 1992;13:296–311. [Google Scholar]
- Saal FE, Downey RG, Lahey MA. Rating the ratings: Assessing the psychometric quality of rating data. Psychological Bulletin. 1980;88:413–428. [Google Scholar]
- Samuels CA, Ewy R. Aesthetic perception of faces during infancy. British Journal of Developmental Psychology. 1985;3:221–228. [Google Scholar]
- Thorndike L. A constant error in psychological findings. Journal of Applied Psychology. 1920;4:25–29. [Google Scholar]
- Valentine T. A unified account of the effects of distinctiveness, inversion, and race on face recognition. Quarterly Journal of Experimental Psychology. 1991;43A:161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- Valentine T, Bruce V. Recognizing familiar faces: The role of distinctiveness and familiarity. Canadian Journal of Psychology. 1986;40:300–305. doi: 10.1037/h0080101. [DOI] [PubMed] [Google Scholar]
- Vokey JR, Read JD. Familiarity, memorability, and the effect of typicality on the recognition of faces. Memory & Cognition. 1992;20:291–302. doi: 10.3758/bf03199666. [DOI] [PubMed] [Google Scholar]
- Wald A. Tests of statistical hypothesis concerning several parameters when the umber of observations is large. Transactions of the American Mathematical Society. 1943;54:426–482. [Google Scholar]
- Walton GE, Bower TGR. Newborns form “prototypes” in less than 1 minute. Psychological Science. 1993;4:203–205. [Google Scholar]
- Wild HA, Barrett SE, Spence MJ, O’Toole AJ, Cheng YD, Brooke J. Recognition and sex categorization of adults’ and children’s faces: Examining performance in the absence of sex-stereotyped cues. Journal of Experimental Child Psychology. 2000;77:269–291. doi: 10.1006/jecp.1999.2554. [DOI] [PubMed] [Google Scholar]
- Wille DE. The 1990s: Gender differences in parenting roles. Sex Roles. 1995;33:803–817. [Google Scholar]
- Winer, B. J., Brown, D. R., & Michels, K. M. (1991). Statistical principles in experimental design. New York, NY: McGraw-Hill.
- Yamaguchi MK, Hirukawa T, Kanazawa S. Judgment of gender through facial parts. Perception. 1995;24:563–575. doi: 10.1068/p240563. [DOI] [PubMed] [Google Scholar]


