Abstract
Hepatitis C virus (HCV), a major etiologic agent of hepatocellular carcinoma, presently infects approximately 400 million people worldwide, making the development of protective measures against HCV infection a key objective. Here we have generated a recombinant vesicular stomatitis virus (VSV), which expresses the HCV structural proteins, by inserting the contiguous Core, E1, and E2 coding region of HCV into the VSV genome. Recombinant VSV expressing HCV Core, E1, and E2 (VSV-HCV-C/E1/E2) grew to high titers in vitro and efficiently expressed the incorporated HCV gene product, which became fully processed into the individual HCV structural proteins. Biochemical and biophysical analysis indicated that the HCV Core, E1, and E2 proteins assembled to form HCV-like particles (HCV-LPs) possessing properties similar to the ultrastructural properties of HCV virions. Mice immunized with VSV-HCV-C/E1/E2 generated cell-mediated immune responses to all of the HCV structural proteins, and humoral responses, particularly to E2, were also readily evident. Our data collectively indicate that engineered VSVs expressing HCV Core, E1, and E2 and/or HCV-LPs represent useful tools in vaccine and immunotherapeutic strategies designed to address HCV infection.
Hepatitis C virus (HCV), a hepatotropic, positive-stranded RNA virus of the Flaviviridae family, is estimated to infect at least 400 million people worldwide and is a major etiologic agent of liver failure and hepatocellular carcinoma (11, 66, 70). HCV comprises a 9.6-kb genome with a conserved 5′ untranslated region that functions as an internal ribosome entry site (33, 69). The untranslated region precedes a long open reading frame that encodes a 3,010-amino-acid (aa) polypeptide that is subsequently cleaved into 10 protein products (33). The amino-terminal region of the viral polypeptide is posttranslationally processed by host cell proteases to generate three structural protein products, Core (nucleocapsid) and envelope glycoproteins E1 and E2 (31, 43). Nonstructural proteins that facilitate virus replication (NS2, -3, - 4a, -4b, -5a, and -5b) reside in the carboxy region of the polypeptide and are cleaved by virus-encoded proteases comprising NS2 and -3 (45, 68).
Standard therapeutic intervention for HCV infection consists of the administration of interferon (IFN) in combination with ribavirin. However, less than 50% of infected patients respond to this regimen and few alternative therapies exist (13, 14, 26, 52). There is presently no tissue culture system to efficiently cultivate HCV, which not only hampers research efforts aimed at elucidating the molecular mechanisms of virus replication but also impedes attempts at producing candidate vaccines and immunotherapies that target HCV-related disease. Consequently, a number of recombinant subunit-based HCV vaccine strategies, involving genetic immunization and purified proteins, have been attempted, as well as virus vectors expressing the HCV envelope glycoproteins (3, 8, 25, 30, 56, 63, 65). Determining an effective vaccination strategy has proven difficult since the type of immune response considered effective for the eradication of HCV infection, including natural resolution of HCV or viral clearance resulting from IFN therapy, is still being elucidated. Furthermore, the heterogeneity between multiple HCV genotypes and the generation of quasispecies indicate that cross-protection between HCV strains may be problematic (18, 20, 21). Nevertheless, studies have indicated that recombinant HCV envelope glycoproteins E1 and E2 are able to elicit protective immunity against homologous virus challenge in chimpanzees, an effect thought to be mediated by the generation of anti-E2-neutralizing antibodies (10). Significant evidence, however, also indicates that early, vigorous and sustained Th1 and multispecific cytotoxic-T-lymphocyte (CTL) responses are further critical for the elimination of HCV infection (12, 17, 67). Collectively, data would therefore indicate that an optimum HCV vaccine or posttherapy strategy should not only induce a potent humoral response to neutralize virus infection but should also elicit a strong, broad-range CTL response to multiple HCV epitopes, to limit virus amplification and spread.
Recently, a procedure for generating replication-competent, negative-stranded vesicular stomatitis virus (VSV) entirely from cDNA was established (40, 72). The genetic malleability of VSV has allowed the development of recombinant VSVs (rVSVs) that express foreign viral proteins to high levels (38, 61). The generation of rVSV has been evaluated in a number of vaccine strategies designed to prevent virus infection. For example, live attenuated VSV expressing the human immunodeficiency virus (HIV) envelope (Env) and core (Gag) proteins has been shown to protect rhesus monkeys from AIDS following challenge with a pathogenic AIDS virus (59). Similarly, VSV expressing either influenza or measles virus hemagglutinin protein conferred resistance to lethal influenza virus or measles virus infection, respectively (58, 60).
Advantages of using an rVSV system for vaccine studies include that the virus is relatively innocuous and that naturally occurring human infections are rare. Accordingly, the apparent seroprevalence of VSV antibodies is generally low within the human population (24, 34, 71). Furthermore, VSV has a simple genetic constitution of only five genes (N, P, M, G, and L) and is unable to undergo reassortment or integration (71). The genetic malleability of VSV indicates that large, multiple inserts of foreign genes can be achieved that are expressed to high levels, without dramatically affecting virus growth (23, 29). VSV has been found to elicit strong humoral and cellular immune responses and is able to elicit both mucosal and systemic immunity (22, 58, 59, 74). These properties warrant the consideration of using VSV as a replication-competent virus strategy in HCV vaccine-related studies.
Generation of rVSV expressing HCV Core, E1, and E2.
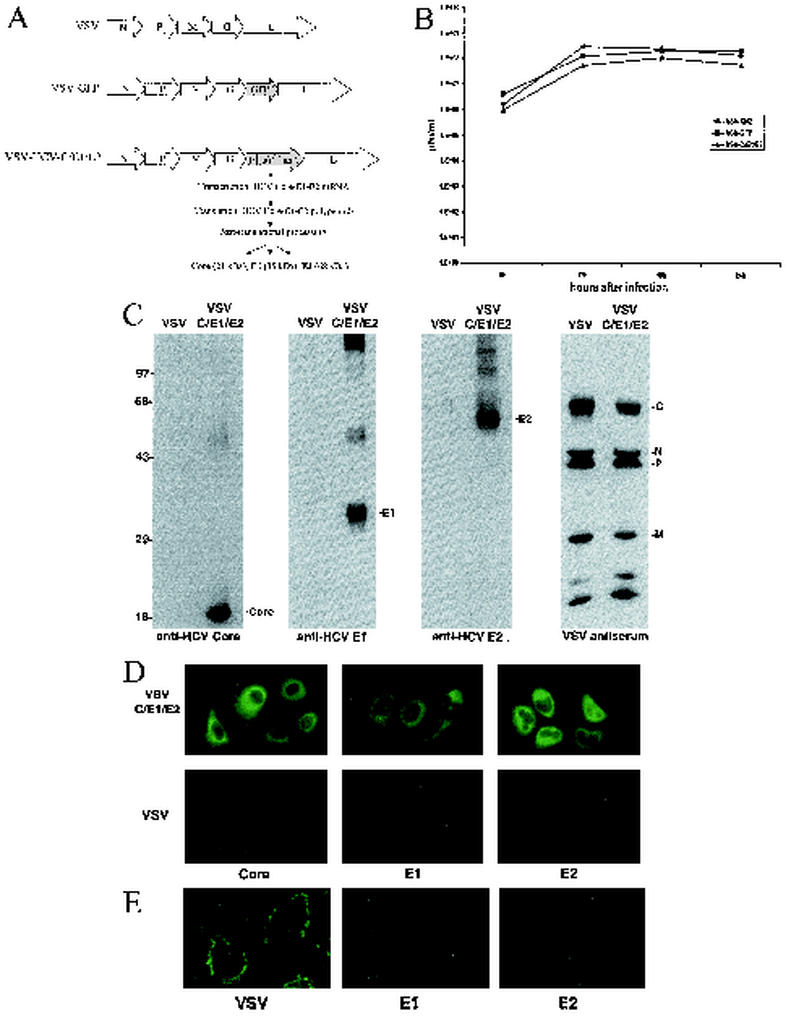
To evaluate whether rVSV could be utilized for the potential development of HCV-related vaccines and immunotherapies, we cloned the entire structural region containing Core, E1, and E2 of HCV genotype 1b (aa 1 to 746; GenBank accession number D89815) into a cDNA representing the VSV genome (pVSV-XN2) (Fig. 1A [2, 62]). To obtain rVSV, the resultant plasmid (pVSV-HCV-C/E1/E2) was transfected into BHK cells with VSV N, P, and L genes and virus was recovered (23). Viable rVSV containing the coding region of the HCV structural proteins (referred to as VSV-HCV-C/E1/E2) was plaque purified and exhibited growth properties similar to those of rVSV expressing green fluorescent protein (VSV-GFP) when examined by one-step growth curve analysis at a starting multiplicity of infection (MOI) of 1 or 0.001 (Fig. 1B and data not shown). To determine whether the recovered rVSV expressed HCV proteins, BHK cells were infected with VSV-XN2 or VSV-HCV-C/E1/E2 at an MOI of 1. Infected cells were lysed 18 h later, and extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To detect HCV proteins, membranes were incubated with anti-Core, -E1, or -E2 antibody. Figure 1C indicates that VSV-HCV-C/E1/E2- but not VSV-XN2-infected cell lysates efficiently expressed all HCV structural proteins. Each of the three HCV products was predominantly detected as correctly processed proteins (Core, 21 kDa; E1, 35 kDa; and E2, 68 kDa), although E2 migrated slightly faster than in studies using other cell types, perhaps due to variations in glycosylation (Fig. 1C [15, 47]). Expression of the VSV proteins was confirmed by Western blot analysis using a polyclonal mouse antiserum to VSV, revealing that the VSV G and M proteins were expressed at lower levels in HCV rVSV, perhaps as a result of the effects of HCV proteins on G and M expression or stability. Confirmation of high-level HCV gene expression was achieved by immunofluorescence analysis of Huh-7 cells infected with control VSV-XN2 or VSV-HCV-C/E1/E2 (Fig. 1D). Antibody raised to HCV core strongly reacted to the perinuclear region of the cell, as previously reported for Core localization in mammalian cells (46, 50). In addition, antibody to E1 and E2 indicated that these HCV glycoproteins resided largely in the cytoplasmic region. This would also be in agreement with previous studies indicating that HCV E1 and E2 form noncovalent heterodimers, which reside as prebudding complexes in the endoplasmic reticulum (ER) of the cell (16, 54). Further immunofluorescence analysis of nonpermeabilized, VSV-HCV-C/E1/E2-infected Huh-7 cells confirmed that E1 and E2 were not expressed on the cell surface (Fig. 1E). Collectively, our data would indicate that VSV can efficiently express HCV Core, E1, and E2 in human cells, which are likely posttranslationally processed in an authentic manner.
FIG. 1.
(A) Construction of rVSV expressing HCV Core, E1, and E2. The Core, E1, and E2 regions (aa 1 to 746) of the HCV polypeptide (NIHJ1 provided by T. Miyamura) were cloned into the XhoI and NheI sites of the rVSV replicon vector pVSV-XN2 (provided by J. Rose) by PCR using the forward primer 5′-CTCGTAGCTCGAGCATCATGAGCACAAATC-3′ and the reverse primer 5′-ACCAAGTTCTCTAGA CTAAGCCTCGGCCTGGGCTAT-39. Recovery of rVSV and the construction of VSV-GFP have been previously described (23). (B) Growth-Growth analysis of recombinant viruses. VSV-HCV-C/E1/E2 demonstrates a growth rate similar to that of rVSV-GFP. BHK cells were infected at an MOI of 1 for 30 min. One hundred microliters of cell medium was collected at 6, 12, 18, and 24 h postinfection and virus titers were determined by plaque assay, as described previously (4). (C) Expression of HCV Core, E1, and E2. BHK cells were infected with VSV-HCV-C/E1/E2 or control virus VSV-XN2 at an MOI of 1. After 18 h, cells were lysed and HCV protein expression was determined by immunoblot analysis as previously described (19). HCV proteins were detected by anti-Core MAb (Biogenesis, Poole, United Kingdom), anti-E1 (a gift from S. J. Polyak), and anti-E2 (WU105; a gift from C. M. Rice). VSV proteins were detected by polyclonal mouse antiserum generated in BALB/c mice infected with VSV. (D) Immunofluorescence analysis of HCV structural proteins. Expression and intracellular localization of HCV structural proteins were confirmed by immunofluorescence using MAbs specific for Core (Biogenesis), E1 (A4; a gift from H. Greenberg), or E2 (544; a gift from M. Kohara). Briefly, Huh-7 cells (provided by S. Lemon) were infected with VSV-XN2 or VSV-HCV-C/E1/E2 at an MOI of 10 for 5 h and were then fixed in 1% paraformaldehyde. The cells were incubated in 1:50 dilutions of primary antibody in 0.1% Brij 97_PBS for 2 h at 4°C and were then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse (1:100; Gibco-BRL, Grand Island, N.Y.) in 0.1% Brij 97_PBS for 1 h at 4°C. (E) Cell surface immunofluorescence staining of VSV-C/E1/E2-infected cells. Immunofluorescence analysis of infected Huh-7 cells was performed as for panel D except that the cells were not permeabilized with Brij 97. VSV-HCV-C/E1/E2-infected cells were stained for cell surface expression of E1 or E2 using MAbs or for expression of VSV using polyclonal mouse serum.
Generation and characterization of HCV-like particles (HCV-LPs).
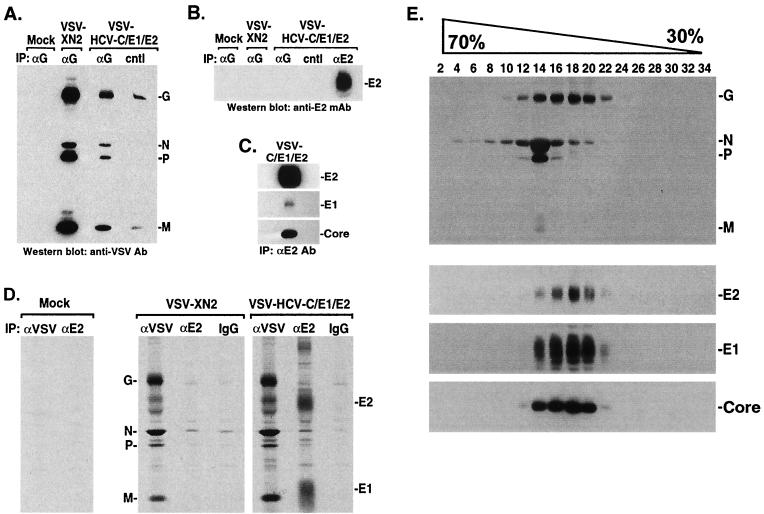
Evidence indicated that viable VSV was effectively generated to express at high levels the HCV structural proteins Core, E1, and E2. To further study HCV Core, E1, and E2 expression and association in mammalian cells through the use of VSV, tissue culture medium or cell lysates from VSV- or VSV-HCV-C/E1/E2-infected BHK cells were clarified by centrifugation and analyzed by immunoprecipitation and immunoblotting for the presence of HCV or VSV proteins. While the majority of proteins detected in the medium appeared to be predominantly VSV related, immunoprecipitation of tissue-cultured medium from S35-labeled, VSV-HCV-C/E1/E2-infected cells using anti-Core or anti-E2 antibody revealed the presence of Core, as well as that of E2 in tight association with E1, suggesting that a proportion of HCV structural proteins was being released from the cell, perhaps as a result of cytolysis (data not shown). To clarify the association of HCV proteins with VSV, medium from VSV-XN2- or VSV-HCV-C/E1/E2-infected cells was immunoprecipitated using a sheep antibody to VSV G. Following washing, complexes were separated by SDS-PAGE and immunoblotted against mouse antiserum raised to VSV. Figure 2A indicates that the VSV structural proteins N, P, and M could be coimmunoprecipitated using the anti-G antibody. However, reprobing the blot with mouse anti-E2 antibody did not reveal detectable E2 protein in medium precipitated with anti-G, indicating that the released E1 and E2 probably did not constitute a physical component of the VSV-HCV-C/E1/E2 virion (Fig. 2B). Precipitation of tissue-cultured medium from VSV-HCV-C/E1/E2-infected cells with goat anti-E2 antibody, followed by immunoblotting with a mouse antibody raised to E2, confirmed the presence of this HCV envelope protein in the medium (Fig. 2B). Thus, although the HCV structural proteins are readily detectable in the medium, these proteins do not appear to be strongly associated with VSV-HCV-C/E1/E2 or to form chimeric viruses. This is most likely due to the HCV envelope products predominantly residing in the ER of the cell in addition to lacking C-terminal regions of VSV G critically required for incorporation into VSV particles as they dissociate from the cell membrane (73).
FIG. 2.
(A and B) HCV E2 is not associated with VSV-HCV-C/E1/E2. Cell medium from VSV-HCV-C/E1/E2- or control virus-infected cells (concentrated by ultracentrifugation) was immunoprecipitated (IP) with a sheep antibody (Ab) to VSV G (Biogenesis) or a goat antibody to HCV E2 (Immunodiagnostics Inc.). After SDS-PAGE, protein complexes were immunoblotted against mouse antiserum raised to VSV (A). Membranes were reprobed using mouse antiserum to HCV E2 (M. Kohara), which emphasized the absence of E2 in VSV complexes (B). cntl represents protein G agarose, which was used as a negative control. (C) Gradient-purified HCV Core, E1, and E2 form complexes. Sucrose gradient fractions containing HCV-LPs were identified by immunoblot and were immunoprecipitated using E2-specific antibody (Immunodiagnostics Inc.). Complexes were washed, analyzed by SDS-PAGE, and immunoblotted using antibody to HCV Core (Biogenesis), E1 (H. Greenberg), and E2 (M. Kohara). (D) Coimmunoprecipitation analysis of VSV-HCV-C/E1/E2-infected cells. [35S]methionine/cysteine-labeled lysates (600 μCi/12 h) from mock, VSV-XN2, or VSV-HCV-C/E1/E2 were immunoprecipitated with mouse antiserum raised to VSV or anti-E2 MAb (544; M. Kohara) or normal mouse immunoglobulin G. Complexes were washed, analyzed by SDS-PAGE, and visualized by autoradiography. (E) Analysis of HCV-LPs by sucrose gradients. BHK cells were infected at an MOI of 0.1 for 18 h and were then lysed in 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 0.5 mM EDTA. The lysates were clarified by centrifugation through 30% sucrose for 6 h at 150,000 × g. The resulting pellets were layered onto a continuous 30 to 70% sucrose gradient. One-milliliter fractions were collected, centrifuged, and analyzed by SDS-PAGE and immunoblotting using antibody to VSV (top gel) or HCV Core (Biogenesis), E1 (S. Polyak), or E2 (C. Rice) (bottom three gels). HCV structural proteins colocalize in fractions 14 to 20 and are separable from VSV proteins.
As indicated by our earlier immunofluorescence and immunoblot studies, HCV structural proteins were predominantly found in the cell lysate fraction rather than the medium (Fig. 1C and D). To explore the association of intracellular HCV and VSV proteins, BHK cells were infected at an MOI of 10 with VSV-XN2 or VSV-HCV-C/E1/E2. Four hours postinfection, cells were labeled with [35S]methionine/cysteine for another 12 h before being lysed. Cell extracts precipitated with a mouse anti-E2 monoclonal antibody (MAb) confirmed strong association of HCV E1 with E2, as may be expected, but not with any VSV proteins (Fig. 2D). Reciprocal coimmunoprecipitation studies using mouse antiserum to VSV also indicated little or no association of HCV E1 and E2 with VSV products, again indicating that HCV proteins are not strongly coupled with VSV complexes (Fig. 2D).
Previous studies have indicated that cooperative expression of Core, E1, and E2 in insect cells using a baculovirus vector resulted in reassembly of the structural proteins to form HCV-LPs (5). To further evaluate the association of the HCV proteins in our system, cell lysates previously infected with VSV-HCV-C/E1/E2 or control VSV-XN2 were clarified by centrifugation, layered onto a continuous 30 to 70% sucrose equilibrium gradient, and centrifuged for 22 h at 150,000 × g. One-milliliter fractions were collected, centrifuged at 150,000 × g, and analyzed by immunoblot for HCV proteins. This study revealed that Core, E1, and E2 collectively sedimented in fractions 14 to 20, strongly suggesting the association of these structural proteins into virus-like particles (VLPs) (Fig. 2E). Similar findings were obtained upon sucrose gradient analysis of medium from VSV-HCV-C/E1/E2-infected cells, indicating that HCV complexes are also released from the cell (data not shown). In contrast, VSV proteins N and P, with small amounts of M and G protein, were found to reside essentially in fraction 14. The small amounts of VSV G in fraction 14 may be explained by reports that the ribonucleocapsid complex of VSV comprising newly synthesized N, P, and L and replicated genomic RNA does not associate with M and G until budding occurs from the cell membrane (71). Thus, the cellular fraction used in this study may predominantly contain VSV ribonucleoprotein complexes rather than entire VSV virions. In contrast, prior studies have demonstrated that E1 and E2 associate as noncovalent heterodimers in the ER, the localization where HCV budding and assembly are believed to occur (15, 57). Collectively, our observations would indicate that HCV Core, E1, and E2 form higher-order structures with one another that can be partially separated from VSV complexes. However, it is interesting that some VSV G was evident in fractions 14 to 20 containing HCV Core, E1, and E2 (Fig. 2E). Therefore, contrary to our previous findings, we cannot presently rule out that residual G protein may be taken up into HCV-LPs prior to or following budding from the ER.
To further analyze the biophysical properties of the HCV-LPs, the densities of the putative VLPs isolated from sucrose gradients were determined. Our results indicated that densities ranged from 1.15 to 1.20 g/ml, which are in accordance with those found for HCV-LPs manufactured in insect cells (1.14 to 1.18 g/ml) (5) and infectious HCV as determined by Kaito et al. (1.12 to 1.17 g/ml) (36) and Bradley et al. (1.09 to 1.21 g/ml) (7). Slight variances from infectious HCV density values may be expected since HCV-LPs lack a packaged genome, as well as any protein that may be associated with it.
Given the supportive data for the formation of HCV-LPs, further biochemical and biophysical studies of HCV-LP formation were undertaken. First, goat anti-HCV E2 antibody was used to immunoprecipitate gradient-purified HCV-LPs (Fig. 2C). After several washes, complexes were resolved using polyacrylamide gels and were transferred to membranes for immunoblotting using anti-HCV Core and E1 mouse antibodies. Figure 2C reveals that HCV Core and E1 (to a lesser extent, which we believe to be due to the weak affinity of the antibody) could be detected in coimmunoprecipitation experiments of gradient-purified putative HCV-LPs using anti-E2 antibody, indicating coassociation of Core and E1 with E2.
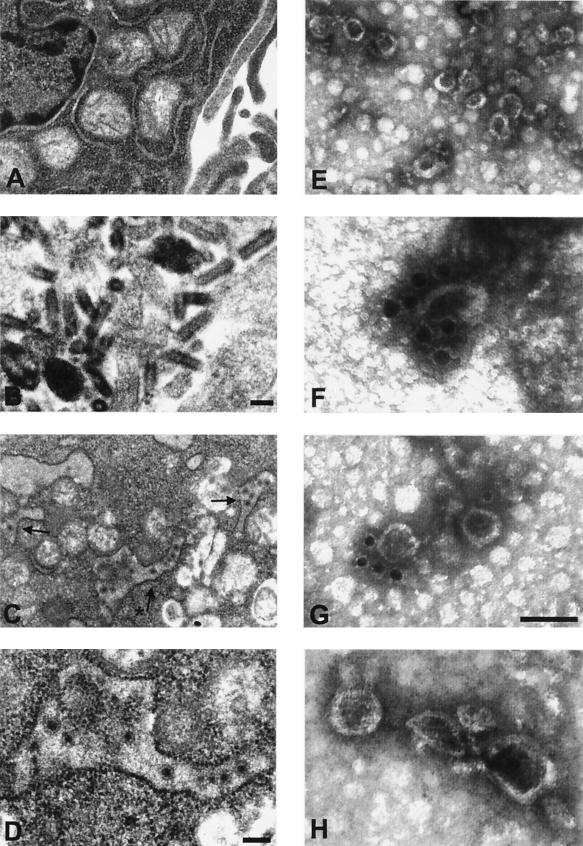
In addition to these studies, uninfected BHK cells or BHK cells infected with either VSV-XN2 or VSV-HCV-C/E1/E2 were analyzed by transmission electron microscopy (TEM) for the potential identification of HCV-LPs (Fig. 3C and D). When cells infected with VSV-HCV-C/E1/E2 but not with VSV-XN2 were examined by TEM, putative HCV-LPs were found in cytoplasmic vacuoles, perhaps generated from rough ER, and appeared to be 40 to 80 nm in dimension. The apparent size would correlate with previous studies analyzing sera of HCV-infected patients, which have calculated hepatitis C virions to be approximately 40 to 70 nm in diameter (36, 42, 64). HCV-LPs morphologically appeared to have a dense core with an evident envelope, while VSV was observed to be characteristically bullet shaped or to consist of a dense outer ring with a transparent core in cross-section analysis (32, 51).
FIG. 3.
(A to D) Electron microscopy images of BHK cells infected with VSV-HCV-C/E1/E2. BHK cells were mock infected or infected at an MOI of 0.1 with VSV-XN2 or VSV-HCV-C/E1/E2 for 18 h. Cells were fixed in 2% paraformaldehyde-2.5% glutaraldehyde and were incubated in 1% osmium tetroxide for 1 h. Fixed cells were then dehydrated and embedded in Spurr's resin. Thin sections were stained in aqueous 4% uranyl acetate for 20 min followed by lead citrate. (A) Mock- infected BHK cells. Magnification is ×19,444. (B) VSV-XN2-infected BHK cells producing the characteristically bullet-shaped VSV virion. Magnification is ×55,163. Bar is 100 nm. (C) VSV-HCV-C/E1/E2-infected BHK cells at ×19,772 magnification. Black arrows indicate HCV-LPs in vacuoles formed from rough ER. (D) Higher magnification of the vacuole containing HCV-LPs in panel C. Asterisk in panel C indicates the magnified vacuole. Bar is 100 nm (magnification is ×44,190). (E to H) Electron microscopy images of HCV-LPs purified by equilibrium sedimentation sucrose gradients. Gradient-purified particles were adsorbed to carbon-coated copper grids and were then negative stained with 2% uranyl acetate for 2 or 3 min (E) (magnification is ×77,333). (F to H) For immunogold labeling, HCV-LPs were incubated with anti-E1 MAb, magnification, ×259,200 (F) (A4; H. Greenberg); anti-E2 MAb, magnification, ×171,428 (G) (544; M. Kohara); or control mouse immunoglobulin G, magnification, ×214,050 (H), and were stained with 2% uranyl acetate. Bar in inset of panel G is 100 nm.
To complement TEM of VSV-HCV-C/E1/E2- or control virus-infected cells, centrifuged HCV-LP fractions from equilibrium sedimentation gradients were also examined by electron microscopy. Gradient fractions from VSV-HCV-C/E1/E2-infected cells but not from control rVSV gradients appeared to contain HCV-LPs with morphology similar to that of HCV observed in previous reports (6, 36, 64). To further validate that the particles were HCV related, gradient fractions were incubated with 1 μl of anti-HCV E1 or E2 MAb, adsorbed to carbon-coated copper grids, and subsequently incubated with secondary antibody conjugated to 15-nm-diameter gold particles (Auroprobe, Piscataway, N.J.). This approach indicated that the surface of the VLPs isolated from fractions containing HCV Core, E1, and E2 proteins interacted with the anti-E1 antibody (Fig. 3F) and anti-E2 antibody (Fig. 3G). Collectively, these data would indicate that VSV can efficiently express HCV structural proteins in mammalian cells that may assemble into HCV-LPs.
Immunogenicity of VSV expressing HCV Core, E1, and E2.
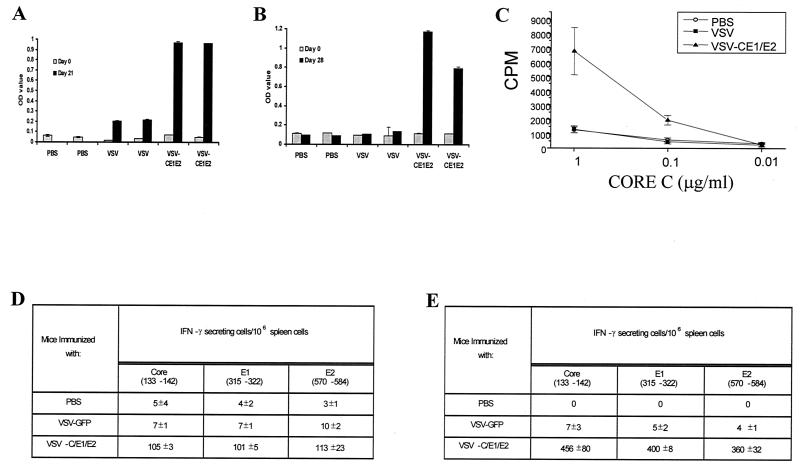
Previous vaccine studies involving rVSV expressing foreign viral antigens have demonstrated potent immune responses to the heterologous product in immunized animals (35, 58-60). In light of these observations, we preliminarily examined in mice whether VSV-HCV-C/E1/E2 could induce an immune response to the HCV structural proteins. BALB/c mice (age, 6 to 8 weeks; Jackson Laboratories) were intravenously (i.v.) injected with 2.5 × 106 PFU of VSV-GFP or VSV-HCV-C/E1/E2 or phosphate-buffered saline (PBS), followed by a second inoculation (5 × 106 PFU, i.v.) 2 weeks later. At 21 days post-initial injection, serum was collected from six mice immunized in each group. Since antibody to E2 has been shown to be critical for neutralization of infectious HCV, we first examined the potential generation of E2 antibodies using enzyme-linked immunosorbent assay (ELISA) and solid-phase E2 protein (Immunodiagnostics, Inc., Woburn, Mass.) as antigen. Each plate containing E2 protein was incubated with several dilutions of mouse serum from PBS-, VSV-GFP-, or VSV-HCV-C/E1/E2-vaccinated mice. Figure 4A indicates that mice vaccinated with VSV-HCV-C/E1/E2 generated a good humoral response to the HCV E2 glycoprotein. Similar observations were demonstrated using HCV core protein as antigen (data not shown). VSV-GFP- vaccinated mice showed a marginal ELISA titer against E2 that may result from minor cross-reactivity by the vigorous anti-VSV antibody response. Collectively, these findings would indicate that VSV-HCV-C/E1/E2 is an efficient vehicle to generate antibodies to HCV envelope E2.
FIG. 4.
Humoral and cellular immune responses generated to HCV structural proteins using VSV as a vector. (A) Humoral activity to HCV E2. ELISA plates (Nunc) adsorbed with E2 protein were used to detect E2 antibody production following i.v. immunization with VSV-HCV-C/E1/E2 but not with VSV-GFP- or PBS-treated control mice (six mice each group) at a dilution of 1/100. Each bar represents a pool of three mice. (B) Humoral activity to HCV E2 generated by i.p. immunization. Anti-E2 ELISAs were conducted on serum collected from mice vaccinated i.p. with VSV-HCV-C/E1/E2, VSV-GFP, or PBS. Each bar represents a single mouse at a dilution of 1/100. OD, optical density. (C) Lymphoproliferative response to HCV. Single-cell preparations from spleens of i.v. immunized mice were suspended in RPMI 1640 supplemented with 10% fetal bovine serum (Sigma, St. Louis, Mo.), 1 mM sodium pyruvate, 100 U of penicillin per ml, and 100 μg of streptomycin (Gibco-BRL) per ml. The cells were stimulated with recombinant HCV core protein (1, 0.1, and 0.01 μg/ml) (Advanced Immunochemical, Long Beach, Calif.). As a negative control, spleen cells were stimulated with medium alone. The wells were pulsed with 1 μCi of [3H]thymidine per well for the last 18 h of a 5-day period. The results are expressed as mean values of triplicate determinations. (D) CTL activation by HCV Core, E1, and E2. IFN-γ production was determined by ELISPOT analysis as previously described (1). Cells harvested from the spleens of i.v. immunized animals (VSV-HCV-C/E1/E2, VSV-GFP, or PBS) were placed in wells in triplicate using four different cell concentrations in twofold dilutions (from 106). These results are representative of three independent experiments, each utilizing a minimum of two mice per group. Each peptide location is noted below the respective HCV protein. (E) IFN-γ ELISPOT analysis of i.p. vaccinated mice. Mice vaccinated i.p. were analyzed for IFN-γ production 7 days following the injection. The results indicate that only VSV-HCV-C/E1/E2-vaccinated mice activate T cells when pulsed with HCV peptides.
To further evaluate the immunogenicity of VSV-HCV-C/E1/E2, lymphoproliferative responses to HCV proteins were also examined. Splenocytes from PBS-, VSV-GFP-, or VSV-HCV-C/E1/E2-vaccinated mice (4 weeks following the initial inoculation of the described protocol) were harvested and pulsed with purified Core or E2 protein. Since exogenous protein should only be presented by major histocompatibility complex class II, only Core- or E2-specific CD4+ T cells should be induced to proliferate. For reasons that remain to be determined, only weak lymphoproliferative responses were observed using E2 protein, perhaps indicating that E2 is not a potent stimulator of CD4+ cells in these animals or due to the instability of the pulsed recombinant E2 protein (data not shown). However, as demonstrated in Fig. 4C, purified Core protein clearly induced the proliferation of T cells from VSV-HCV-C/E1/E2-vaccinated mice but not VSV-GFP- or PBS-inoculated mice.
The generation of a multispecific CTL response is also considered to be important for the clearance of HCV during acute infections in humans and chimpanzees (12, 17, 67). Therefore, to determine if VSV-HCV-C/E1/E2 was able to generate CD8+-T-cell responses to the structural proteins of HCV, gamma IFN (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed on splenocytes isolated from PBS, VSV-GFP, or VSV-HCV-C/E1/E2 4 weeks following the initial vaccination as previously described. Splenocytes from vaccinated mice were pulsed with 10 μg of Core, E1, or E2 peptide per ml, corresponding to HCV genotype 1b, which has been previously shown to activate CTLs in immunized BALB/c mice (27, 53). As shown in Fig. 4D, only CTLs from VSV-HCV-C/E1/E2-vaccinated mice were activated by the HCV-specific peptides as demonstrated by the production of IFN-γ. These data would indicate that intravenously administered VSV-HCV-C/E1/E2 is able not only to induce humoral activity for HCV proteins but also to stimulate CTL activity for the structural proteins of HCV.
As the route of inoculation can affect both the strength and type of immunity generated, we also examined the primary response generated by intraperitoneal (i.p.) inoculation of 5 × 107 PFU of VSV-GFP, VSV-HCV-C/E1/E2, or PBS. Serum was collected 7 and 28 days postinoculation, and ELISAs were performed for anti-E2 antibody production. By day 28, significant anti-E2 antibody levels were detected in VSV-HCV-C/E1/E2-immunized mice (Fig. 4B). Furthermore, 7 days after mice received VSV-HCV-C/E1/E2, splenocytes from vaccinated animals were analyzed for IFN-γ production following stimulation with HCV-specific peptides. Figure 4E demonstrates that only VSV-HCV-C/E1/E2-infected mice contained evidence of CTLs to HCV structural proteins following vaccination,
In conclusion, we have reported the generation of rVSV that expresses the HCV genotype 1b structural proteins Core, E1, and E2. The growth of the recombinant viruses was comparable to that of wild-type rVSV and expressed high levels of recombinant HCV proteins. Recent reports also described the successful generation of rVSV, non-rVSV, or recombinant rabies virus expressing the envelope glycoproteins of HCV on the surface of the virion, though not with Core (9, 39, 48, 49, 65). The expression of Core, E1, and E2 using VSV in mammalian cells resulted in efficient cleavage of the polyprotein precursor and authentic modification of the Core and E1 proteins. Accordingly, the host environment also facilitated assembly of the HCV proteins into VLPs, as determined both biophysically and biochemically. Similar results have been reported following expression of HCV Core, E1, and E2 in insect cells using a baculovirus vector and in cells infected with a Sendai virus replicon containing HCV proteins (5, 6). Insect cell-expressed VLPs have been shown to stimulate humoral and cellular immune responses in mice in the absence of adjuvant. Their ability to provoke these responses is likely dependent on the structural integrity of the particle formation. The conformation of VLPs presumably enhances uptake by professional antigen-presenting cells and processing by the major histocompatibility complex class I pathway to stimulate CTL activity. Both humoral and cellular responses were elicited by VLPs against multiple viral proteins simultaneously, indicating that a strong, broad-range immune response against multiple HCV targets may be feasible (41). Similarly, the stimulation of CTL activity has been observed using recombinant HIV VLPs and human papilloma-VLPs in animal studies (28, 37, 44, 55). It presently remains to be determined whether VSV-synthesized HCV-LPs alone are able to stimulate both broad-range humoral and cell-mediated activity to HCV proteins.
In addition to being an efficient vector for the expression of HCV-LPs in mammalian cells, VSV-HCV-C/E1/E2 affords the opportunity of evaluation as a vaccine candidate itself. VSV expressing HIV proteins has been shown to protect against a pathogenic HIV-like virus, influenza virus, and measles virus (58-60). We demonstrate here that low doses of VSV-HCV-C/E1/E2 could efficiently generate humoral and CTL activity to the structural proteins of HCV. Studies in our laboratory have indicated that these rVSVs are extremely attenuated and that no overt anomalies have been observed in BALB/c animals receiving 5 × 107 PFU of rVSVs i.v. (data not shown). Thus, further work is presently under way to additionally evaluate and optimize the efficacy of VSV-HCV constructs as well as HCV-LPs in vaccine and immunotherapeutic strategies designed to combat HCV infection.
Acknowledgments
We thank J. Rose and T. Miyamura for providing the constructs pVSV-XN2 and pNIHJ1 (HCV 1b), respectively. In addition, we are greatly appreciative for the following antibodies provided: anti-E1 (provided by Steven J. Polyak and Harry Greenberg [A4]) and anti-E2 (provided by Charles M. Rice [WU105] and Michinori Kohara [544]). We also thank Stanley Lemon for providing Huh-7 cells.
REFERENCES
- 1.Adkins, B., Y. Bu, and P. Guevara. 2001. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 166:918-925. [DOI] [PubMed] [Google Scholar]
- 2.Aizaki, H., Y. Aoki, T. Harada, K. Ishii, T. Suzuki, S. Nagamori, G. Toda, Y. Matsuura, and T. Miyamura. 1998. Full-length complementary DNA of hepatitis C virus genome from an infectious blood sample. Hepatology 27:621-627. [DOI] [PubMed] [Google Scholar]
- 3.Arichi, T., T. Saito, M. E. Major, I. M. Belyakov, M. Shirai, V. H. Engelhard, S. M. Feinstone, and J. A. Berzofsky. 2000. Prophylactic DNA vaccine for hepatitis C virus (HCV) infection: HCV-specific cytotoxic T lymphocyte induction and protection from HCV-recombinant vaccinia infection in an HLA-A2.1 transgenic mouse model. Proc. Natl. Acad. Sci. USA 97:297-302. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 74:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumert, T. F., S. Ito, D. T. Wong, and T. J. Liang. 1998. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J. Virol. 72:3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard, E., D. Brand, S. Trassard, A. Goudeau, and P. Roingeard. 2002. Hepatitis C virus-like particle morphogenesis. J. Virol. 76:4073-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley, D., K. McCaustland, K. Krawczynski, J. Spelbring, C. Humphrey, and E. H. Cook. 1991. Hepatitis C virus: buoyant density of the factor VIII-derived isolate in sucrose. J. Med. Virol. 34:206-208. [DOI] [PubMed] [Google Scholar]
- 8.Brinster, C., S. Muguet, Y. C. Lone, D. Boucreux, N. Renard, A. Fournillier, F. Lemonnier, and G. Inchauspe. 2001. Different hepatitis C virus nonstructural protein 3 (Ns3)-DNA-expressing vaccines induce in HLA-A2.1 transgenic mice stable cytotoxic T lymphocytes that target one major epitope. Hepatology 34:1206-1217. [DOI] [PubMed] [Google Scholar]
- 9.Buonocore, L., K. J. Blight, C. M. Rice, and J. K. Rose. 2002. Characterization of vesicular stomatitis virus recombinants that express and incorporate high levels of hepatitis C virus glycoproteins. J. Virol. 76:6865-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choo, Q. L., G. Kuo, R. Ralston, A. Weiner, D. Chien, G. Van Nest, J. Han, K. Berger, K. Thudium, C. Kuo, et al. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 91:1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 13.Davis, G. L., R. Esteban-Mur, V. Rustgi, J. Hoefs, S. C. Gordon, C. Trepo, M. L. Shiffman, S. Zeuzem, A. Craxi, M. H. Ling, J. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N. Engl. J. Med. 339:1493-1499. [DOI] [PubMed] [Google Scholar]
- 14.Dieterich, D. T., J. M. Purow, and R. Rajapaksa. 1999. Activity of combination therapy with interferon alfa-2b plus ribavirin in chronic hepatitis C patients co-infected with HIV. Semin. Liver Dis. 19:87-94. [PubMed] [Google Scholar]
- 15.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubuisson, J., and C. M. Rice. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 70:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 18.Esumi, M., T. Rikihisa, S. Nishimura, J. Goto, K. Mizuno, Y. H. Zhou, and T. Shikata. 1999. Experimental vaccine activities of recombinant E1 and E2 glycoproteins and hypervariable region 1 peptides of hepatitis C virus in chimpanzees. Arch. Virol. 144:973-980. [DOI] [PubMed] [Google Scholar]
- 19.Ezelle, H. J., S. Balachandran, F. Sicheri, S. J. Polyak, and G. N. Barber. 2001. Analyzing the mechanisms of interferon-induced apoptosis using CrmA and hepatitis C virus NS5A. Virology 281:124-137. [DOI] [PubMed] [Google Scholar]
- 20.Farci, P., H. J. Alter, S. Govindarajan, D. C. Wong, R. Engle, R. R. Lesniewski, I. K. Mushahwar, S. M. Desai, R. H. Miller, N. Ogata, et al. 1992. Lack of protective immunity against reinfection with hepatitis C virus. Science 258:135-140. [DOI] [PubMed] [Google Scholar]
- 21.Farci, P., A. Shimoda, D. Wong, T. Cabezon, D. De Gioannis, A. Strazzera, Y. Shimizu, M. Shapiro, H. J. Alter, and R. H. Purcell. 1996. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 93:15394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehr, T., M. F. Bachmann, H. Bluethmann, H. Kikutani, H. Hengartner, and R. M. Zinkernagel. 1996. T-independent activation of B cells by vesicular stomatitis virus: no evidence for the need of a second signal. Cell. Immunol. 168:184-192. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez, M., M. Porosnicu, D. Markovic, and G. N. Barber. 2002. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J. Virol. 76:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields, B. N., and K. Hawkins. 1967. Human infection with the virus of vesicular stomatitis during an epizootic. N. Engl. J. Med. 277:989-994. [DOI] [PubMed] [Google Scholar]
- 25.Forns, X., P. J. Payette, X. Ma, W. Satterfield, G. Eder, I. K. Mushahwar, S. Govindarajan, H. L. Davis, S. U. Emerson, R. H. Purcell, and J. Bukh. 2000. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology 32:618-625. [DOI] [PubMed] [Google Scholar]
- 26.Gerotto, M., D. G. Sullivan, S. J. Polyak, L. Chemello, L. Cavalletto, P. Pontisso, A. Alberti, and D. R. Gretch. 1999. Effect of retreatment with interferon alone or interferon plus ribavirin on hepatitis C virus quasispecies diversification in nonresponder patients with chronic hepatitis C. J. Virol. 73:7241-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon, E. J., R. Bhat, Q. Liu, Y. F. Wang, C. Tackney, and A. M. Prince. 2000. Immune responses to hepatitis C virus structural and nonstructural proteins induced by plasmid DNA immunizations. J. Infect. Dis. 181:42-50. [DOI] [PubMed] [Google Scholar]
- 28.Greenstone, H. L., J. D. Nieland, K. E. de Visser, M. L. De Bruijn, R. Kirnbauer, R. B. Roden, D. R. Lowy, W. M. Kast, and J. T. Schiller. 1998. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA 95:1800-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haglund, K., J. Forman, H. G. Krausslich, and J. K. Rose. 2000. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology 268:112-121. [DOI] [PubMed] [Google Scholar]
- 30.Heile, J. M., Y.-L. Fong, D. Rosa, K. Berger, G. Saletti, S. Campagnoli, G. Bensi, S. Capo, S. Coates, K. Crawford, C. Dong, M. Wininger, G. Baker, L. Cousens, D. Chien, P. Ng, P. Archangel, G. Grandi, M. Houghton, and S. Abrignani. 2000. Evaluation of hepatitis C virus glycoprotein E2 for vaccine design: an endoplasmic reticulum-retained recombinant protein is superior to secreted recombinant protein and DNA-based vaccine candidates. J. Virol. 74:6885-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hijikata, M., N. Kato, Y. Ootsuyama, M. Nakagawa, and K. Shimotohno. 1991. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc. Natl. Acad. Sci. USA 88:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland, J. J. 1987. Defective interfering rhabdoviruses, p. 297-360. In R. R. Wagner (ed.), The rhabdoviruses. Plenum, New York, N.Y.
- 33.Houghton, M. 1996. Hepatitis C viruses, p. 1035-1058. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 34.Johnson, K. M., J. E. Vogel, and P. H. Peralta. 1966. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV). Am. J. Trop. Med. Hyg. 15:244-246. [DOI] [PubMed] [Google Scholar]
- 35.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaito, M., S. Watanabe, K. Tsukiyama-Kohara, K. Yamaguchi, Y. Kobayashi, M. Konishi, M. Yokoi, S. Ishida, S. Suzuki, and M. Kohara. 1994. Hepatitis C virus particle detected by immunoelectron microscopic study. J. Gen. Virol. 75:1755-1760. [DOI] [PubMed] [Google Scholar]
- 37.Kang, C. Y., L. Luo, M. A. Wainberg, and Y. Li. 1999. Development of HIV/AIDS vaccine using chimeric gag-env virus-like particles. Biol. Chem. 380:353-364. [DOI] [PubMed] [Google Scholar]
- 38.Kretzschmar, E., L. Buonocore, M. J. Schnell, and J. K. Rose. 1997. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J. Virol. 71:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagging, L. M., K. Meyer, R. J. Owens, and R. Ray. 1998. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J. Virol. 72:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lechmann, M., K. Murata, J. Satoi, J. Vergalla, T. F. Baumert, and T. J. Liang. 2001. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology 34:417-423. [DOI] [PubMed] [Google Scholar]
- 42.Li, X., L. J. Jeffers, L. Shao, K. R. Reddy, M. de Medina, J. Scheffel, B. Moore, and E. R. Schiff. 1995. Identification of hepatitis C virus by immunoelectron microscopy. J. Viral Hepat. 2:227-234. [DOI] [PubMed] [Google Scholar]
- 43.Lin, C., B. D. Lindenbach, B. M. Pragai, D. W. McCourt, and C. M. Rice. 1994. Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J. Virol. 68:5063-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, X. S., I. Abdul-Jabbar, Y. M. Qi, I. H. Frazer, and J. Zhou. 1998. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology 252:39-45. [DOI] [PubMed] [Google Scholar]
- 45.Lohmann, V., J. O. Koch, and R. Bartenschlager. 1996. Processing pathways of the hepatitis C virus proteins. J. Hepatol. 24:11-19. [PubMed] [Google Scholar]
- 46.Martire, G., A. Viola, L. Iodice, L. V. Lotti, R. Gradini, and S. Bonatti. 2001. Hepatitis C virus structural proteins reside in the endoplasmic reticulum as well as in the intermediate compartment/cis-Golgi complex region of stably transfected cells. Virology 280:176-182. [DOI] [PubMed] [Google Scholar]
- 47.Matsuura, Y., T. Suzuki, R. Suzuki, M. Sato, H. Aizaki, I. Saito, and T. Miyamura. 1994. Processing of E1 and E2 glycoproteins of hepatitis C virus expressed in mammalian and insect cells. Virology 205:141-150. [DOI] [PubMed] [Google Scholar]
- 48.Matsuura, Y., H. Tani, K. Suzuki, T. Kimura-Someya, R. Suzuki, H. Aizaki, K. Ishii, K. Moriishi, C. S. Robison, M. A. Whitt, and T. Miyamura. 2001. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology 286:263-275. [DOI] [PubMed] [Google Scholar]
- 49.Meyer, K., A. Basu, and R. Ray. 2000. Functional features of hepatitis C virus glycoproteins for pseudotype virus entry into mammalian cells. Virology 276:214-226. [DOI] [PubMed] [Google Scholar]
- 50.Moradpour, D., P. Kary, C. M. Rice, and H. E. Blum. 1998. Continuous human cell lines inducibly expressing hepatitis C virus structural and nonstructural proteins. Hepatology 28:192-201. [DOI] [PubMed] [Google Scholar]
- 51.Nakai, T., and A. F. Howatson. 1968. The fine structure of vesicular stomatitis virus. Virology 35:268-281. [DOI] [PubMed] [Google Scholar]
- 52.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura, Y., A. Kamei, S. Uno-Furuta, S. Tamaki, G. Kim, Y. Adachi, K. Kuribayashi, Y. Matsuura, T. Miyamura, and Y. Yasutomi. 1999. A single immunization with a plasmid encoding hepatitis C virus (HCV) structural proteins under the elongation factor 1-alpha promoter elicits HCV-specific cytotoxic T-lymphocytes (CTL). Vaccine 18:675-680. [DOI] [PubMed] [Google Scholar]
- 54.Op De Beeck, A., R. Montserret, S. Duvet, L. Cocquerel, R. Cacan, B. Barberot, M. Le Maire, F. Penin, and J. Dubuisson. 2000. The transmembrane domains of hepatitis C virus envelope glycoproteins E1 and E2 play a major role in heterodimerization. J. Biol. Chem. 275:31428-31437. [DOI] [PubMed] [Google Scholar]
- 55.Paliard, X., Y. Liu, R. Wagner, H. Wolf, J. Baenziger, and C. M. Walker. 2000. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res. Hum. Retrovir. 16:273-282. [DOI] [PubMed] [Google Scholar]
- 56.Pancholi, P., Q. Liu, N. Tricoche, P. Zhang, M. E. Perkus, and A. M. Prince. 2000. DNA prime-canarypox boost with polycistronic hepatitis C virus (HCV) genes generates potent immune responses to HCV structural and nonstructural proteins. J. Infect. Dis. 182:18-27. [DOI] [PubMed] [Google Scholar]
- 57.Ralston, R., K. Thudium, K. Berger, C. Kuo, B. Gervase, J. Hall, M. Selby, G. Kuo, M. Houghton, and Q. L. Choo. 1993. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J. Virol. 67:6753-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 60.Schlereth, B., J. K. Rose, L. Buonocore, V. ter Meulen, and S. Niewiesk. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schnell, M. J., L. Buonocore, E. Kretzschmar, E. Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seong, Y. R., S. Choi, J. S. Lim, C. H. Lee, C. K. Lee, and D. S. Im. 2001. Immunogenicity of the E1E2 proteins of hepatitis C virus expressed by recombinant adenoviruses. Vaccine 19:2955-2964. [DOI] [PubMed] [Google Scholar]
- 64.Shimizu, Y. K., S. M. Feinstone, M. Kohara, R. H. Purcell, and H. Yoshikura. 1996. Hepatitis C virus: detection of intracellular virus particles by electron microscopy. Hepatology 23:205-209. [DOI] [PubMed] [Google Scholar]
- 65.Siler, C. A., J. P. McGettigan, B. Dietzschold, S. K. Herrine, J. Dubuisson, R. J. Pomerantz, and M. J. Schnell. 2002. Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology 292:24-34. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka, H., T. Hiyama, H. Tsukuma, I. Fujimoto, H. Yamano, Y. Okubo, and A. Kitada. 1994. Cumulative risk of hepatocellular carcinoma in hepatitis C virus carriers: statistical estimations from cross-sectional data. Jpn. J. Cancer Res. 85:485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomei, L., C. Failla, E. Santolini, R. De Francesco, and N. La Monica. 1993. NS3 is a serine protease required for processing of hepatitis C virus polyprotein. J. Virol. 67:4017-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsukiyama-Kohara, K., N. Iizuka, M. Kohara, and A. Nomoto. 1992. Internal ribosome entry site within hepatitis C virus RNA. J. Virol. 66:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsukuma, H., T. Hiyama, S. Tanaka, M. Nakao, T. Yabuuchi, T. Kitamura, K. Nakanishi, I. Fujimoto, A. Inoue, H. Yamazaki, et al. 1993. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N. Engl. J. Med. 328:1797-1801. [DOI] [PubMed] [Google Scholar]
- 71.Wagner, R. R. and J. K. Rose. 1996. Rhabdoviridae: the viruses and their replication, p. 1121-1135. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 72.Whelan, S. P., L. A. Ball, J. N. Barr, and G. T. Wertz. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92:8388-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitt, M. A., L. Chong, and J. K. Rose. 1989. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J. Virol. 63:3569-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zinkernagel, R. M., B. Adler, and J. J. Holland. 1978. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp. Cell Biol. 46:53-70. [DOI] [PubMed] [Google Scholar]