Abstract
The Hedgehog (Hh) family of signalling proteins control both differentiation and proliferation during animal development. Previous studies have shown that Hh signalling has a stimulatory effect on the cell cycle in several organs by controlling core cell-cycle components. Here, we show that Sonic hedgehog (Shh) signalling has the opposite effect in the zebrafish retina, where it leads to cell-cycle exit, and that this is mediated by transcriptional activation of the cyclin kinase inhibitor p57Kip2. The loss of p57Kip2 activity strongly resembles the Shh mutant eye phenotype, and overexpression of p57Kip2 rescues cell-cycle exit in Shh mutants, indicating that p57Kip2 is both necessary and sufficient to mediate Shh-induced cell-cycle exit in the retina. These findings raise the possibility that stimulation of cell-cycle exit through regulation of core cell-cycle components may be part of a general mechanism required for Hh-directed differentiation.
Keywords: cyclin kinase inhibitor, p57Kip2, retina, Sonic hedgehog, zebrafish
Introduction
The vertebrate retina is made up of seven principal cell types, generated in a characteristic order during development (reviewed by Livesey & Cepko, 2001). This process involves tight coordination of proliferation and differentiation, as the number of cell cycles per precursor cell is an important determinant of organ size, whereas cell-cycle exit of precursor cells at the correct time and correct location are necessary for differentiation of appropriate numbers of mature cell types (reviewed by Dyer & Cepko, 2001b; Ohnuma & Harris, 2003).
The Hedgehog (Hh) family of secreted signalling proteins regulates pattern formation, proliferation and differentiation in many developing organs, both in vertebrate and invertebrate embryos (reviewed by Ingham & McMahon, 2001). In several contexts, activation of the Hhsignalling cascade leads to stimulation of proliferation (reviewed by Roy & Ingham, 2002). In mouse cerebellar neuronal precursors, this effect seems to be mediated by the transcriptional upregulation of cyclins D1 and D2 (Kenney & Rowitch, 2000), suggesting that Hh signalling stimulates the cell cycle by regulating core cell-cycle activators. In other contexts, Hh signalling is required for differentiation, and this clearly depends on cell-cycle withdrawal of precursor cells. So far, however, it is not known whether this effect is also mediated through the regulation of the cell cycle.
A well-characterized context in which Hh signalling directs differentiation is the zebrafish retina (reviewed by Stadler et al, 2004). Sonic hedgehog (Shh) is expressed in a subset of retinal ganglion cells and amacrine cells, and is required for differentiation of all principal retinal cell types (Neumann & Nuesslein-Volhard, 2000; Shkumatava et al, 2004). Shh functions as a short-range signal secreted by differentiated neurons, triggering differentiation in nearby progenitor cells and thereby propagating a wave of differentiation through the retinal epithelium.
Cyclin kinase inhibitors (CKIs) of the Cip/Kip family have been shown to trigger cell-cycle exit in several contexts. In the mouse retina, the Kip family members p27Kip1 and p57Kip2 regulate cell-cycle exit in distinct progenitor cell populations (Dyer & Cepko, 2000, 2001a). The mouse p57Kip2 gene is expressed in about 16.5% of retinal progenitor cells, and in the absence of p57Kip2 activity, these progenitor cells continue to proliferate (Dyer & Cepko, 2000).
Here, we show that Shh activity is required for cell-cycle exit of progenitor cells in the zebrafish retina, and that this effect is mediated by p57Kip2. Zebrafish p57Kip2 is transiently expressed in most of the wild-type retinal progenitor cells before they differentiate, but fails to be activated in shh mutants. In the absence of either shh or p57Kip2 activity, most of the retinal progenitor cells continue to proliferate and fail to differentiate. This overproliferation is compensated for by apoptosis. Conversely, overexpression of p57Kip2 is sufficient to rescue cell-cycle exit in shh mutants, indicating that p57Kip2 is both necessary and sufficient to mediate Shh-triggered cell-cycle exit in the zebrafish retina.
Results
Shh is required for cell-cycle exit in the zebrafish retina
To analyse how Shh triggers retinal differentiation, we examined whether cell-cycle exit is perturbed in shh mutants. We assayed cell proliferation with 5-bromodeoxyuridine (BrdU) incorporation, and compared wild type and shh mutants. At 36 hours post fertilization (hpf), we detected the first cells in the ventronasal retina, which had stopped proliferating and expressed markers for retinal ganglion cell differentiation; however, the remaining retina was still strongly labelled with BrdU (Fig 1A). At this stage, no difference is observed between wild type and shh mutants (Fig 1A,B). At 72 hpf, only cells at the retinal margin still proliferated in wild-type embryos, and cells in the central retina had differentiated (Fig 1C). In contrast, a large number of proliferating cells were still present throughout the shh−/− retina (Fig 1D; supplementary Fig 1A online), and there was only a small increase in differentiated retinal ganglion cells (RGCs) compared with the earlier time point (supplementary Fig 1B online). We also examined the number of mitotic cells in the retina with an antibody recognizing the phosphorylated form of histone H3, and observed a greatly increased number in shh mutants compared with wild-type embryos at 72 hpf (Fig 1E,F).
Figure 1.
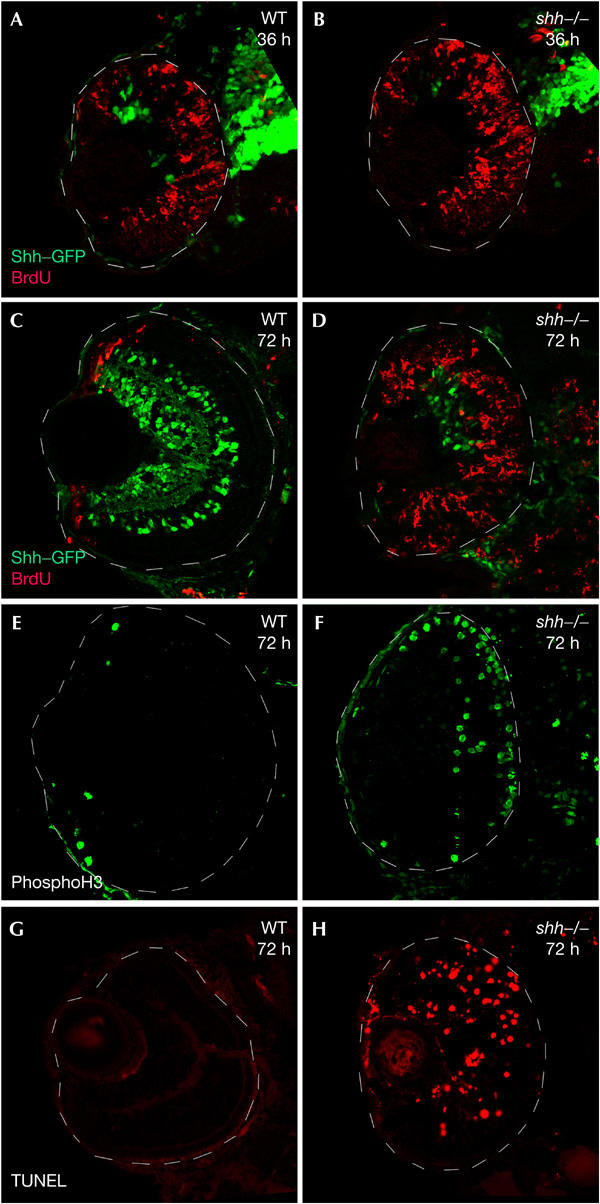
Overproliferation and increased apoptosis in the shh−/− retina. Confocal sections are anterior to the top. Shh–GFP (A–D, green) and BrdU incorporation (A–D, red) are shown. (A,B) BrdU incorporation from 34 hpf to 36 hpf is similar in the wild-type retina (A) and shh mutants (B). (C) BrdU incorporation (70–72 hpf) shows that wild-type embryos have restricted proliferation to the retinal margin at this stage, whereas shh mutants have proliferating cells throughout the retina (D). (E,F) Mitotic cells labelled with anti-phosphohistone H3. Only a few mitotic cells are present at the retinal margin in wild-type embryos at 72 hpf (E). shh mutants have an increased number of mitotic cells (F). (G,H) A TUNEL assay performed on wild type (G) and shh mutants (H) to detect apoptotic nuclei at 72 hpf shows a marked increase of apoptosis in shh mutants.
The eyes of shh mutants are smaller than wild-type eyes, suggesting that the excess proliferation in the shh retina must be compensated by apoptosis. Indeed, TdT-mediated dUTP nick end-labelling (TUNEL) staining showed the first apoptotic cells around 48 hpf in the mutant retina (data not shown) and a large number of cells undergoing apoptosis at 72 hpf, whereas no apoptotic cells are detectable in the wild-type retina at the same stages (Fig 1G,H). Taken together, these results indicate that Shh is required for cell-cycle exit of retinal progenitor cells, which in the absence of Shh activity continue to proliferate, and then undergo apoptosis.
In a previous study, it was reported that only half of shh mutants show a strong differentiation defect in the retina (Stenkamp et al, 2002), whereas the other half show much milder defects. We did not see this effect in most genetic backgrounds, and instead observed the strong phenotype consistently (data not shown), suggesting that the milder phenotype might be due to the presence of a secondsite suppressor.
Shh is required for p57Kip2 expression in the retina
To investigate the downstream mechanism whereby Shh directs cell-cycle exit, we analysed the expression of several cell-cycle components. We found that the CKI p57Kip2 and cyclins D1 and E2 are expressed in the developing retina. p57Kip2 was transiently and dynamically expressed in the retina. We detected no expression at the start of neurogenesis in the ventronasal retina (data not shown). Several hours after the first neurons were born, p57Kip2 was expressed in a domain surrounding the region of differentiated neurons in the central retina (Fig 2A). As differentiation proceeds from the central to the peripheral retina, the domain of p57Kip2 transcription also moves to the periphery (Fig 2C). As p57Kip2 has been shown to trigger cell-cycle exit, we examined p57Kip2 expression in shh mutants. No p57Kip2 transcript was detectable in the retina of shh mutants at all stages examined, whereas it was expressed in other tissues of these mutants (Fig 2B,D), indicating that Shh is required for transcriptional activation of p57Kip2 in the retina. We also examined the expression of p27Kip1 (Thisse et al, 2001), but found that it had a weak ubiquitous expression pattern in the central nervous system and is therefore unlikely to be regulated by Shh at the transcriptional level.
Figure 2.
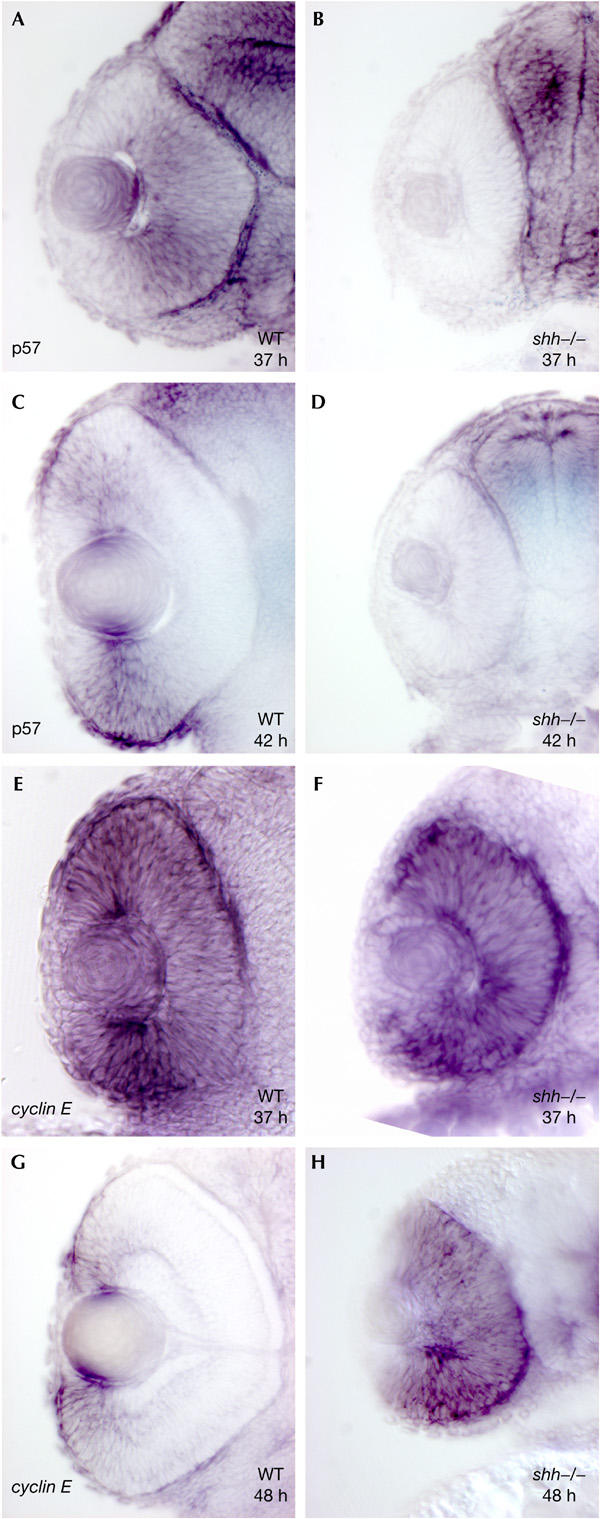
Shh is required for upregulation of p57Kip2 and downregulation of cyclin E2. (A) p57Kip2 transcript is expressed in a domain surrounding the central retina of wild-type embryos at 37 hpf. (C) At 42 hpf, the p57Kip2 expression domain has moved towards the retinal margin. (B,D) shh mutants lack p57Kip2 expression at any stage. (E,F) At 37 hpf, cyclin E2 is expressed in the central retina of wild-type (E) and shh−/− embryos (F). (G) At 48 hpf, cyclin E2 is expressed at the margin in the wild-type retina. (H) shh mutants fail to downregulate cyclin E2 expression in the central retina at 48 hpf.
Cyclins D1 and E2 are positive regulators of the cell cycle, and we detected transcripts for these genes in proliferating precursor cells of the zebrafish retina, but not in differentiated cells (Fig 2E,G; supplementary Fig 1C,E online). Their transcripts were initially present throughout the entire retinal neuroepithelium, and then progressively restricted to the margin as cells in the central retina differentiated (Fig 2G; supplementary Fig 1E online). In contrast, shh−/− embryos failed to downregulate expression of cyclins D1 and E2 in the central retina, and high levels of transcript were still present throughout the entire retina at late stages in shh mutants (Fig 2F,H; supplementary Fig 1D,F online). Taken together, these findings indicate that Shh activity is necessary for the activation of p57Kip2 transcription and for repression of cyclins D1 and E2 transcription in the retina.
p57Kip2 is required for cell-cycle exit in the retina
To test a potential role of p57Kip2 in regulating cell-cycle exit downstream of Shh signalling, we reduced p57Kip2 levels by targeted knockdown using morpholino oligonucleotide injection into oocytes (Nasevicius & Ekker, 2000). We observed phenotypes in several organs in which p57Kip2 was expressed, but initially focused our analysis on the retina. We found that in p57Kip2 morpholino-injected embryos, most of the progenitor cells failed to exit the cell cycle, as indicated by BrdU labelling, but not in embryos injected with control morpholino at the same concentration (Fig 3A,B; data not shown). The number of differentiated RGCs expressing shh–GFP (green fluorescent protein) was severely reduced in p57Kip2 morpholino-injected embryos (Fig 3A,B,E,F), and we failed to detect Islet-1 (Isl1)-expressing amacrine cells, bipolar cells and horizontal cells (Fig 3E,F). There was also a complete absence of Zpr1-expressing photoreceptors (Fig 3G,H) and of Mueller glia, as detected by glutamine synthetase expression at 72 hpf (Fig 3C,D). We were unable to examine later time points because the embryos died soon after 72 hpf. In addition, there was no sign of lamination in morpholino-injected embryos, assayed with phalloidin staining (Fig 3I,J). To test whether p57Kip2 knockdown also leads to apoptosis, we performed TUNEL labelling, and observed an increase in apoptotic cells (Fig 3K,L). Taken together, these results indicate that p57Kip2 activity is necessary for cell-cycle exit and for differentiation in the zebrafish retina. The retinal phenotype of p57Kip2 knockdown embryos strongly resembles that of shh mutants (Fig 1; Shkumatava et al, 2004), suggesting that p57Kip2 is an essential mediator of Shh function in the retina.
Figure 3.
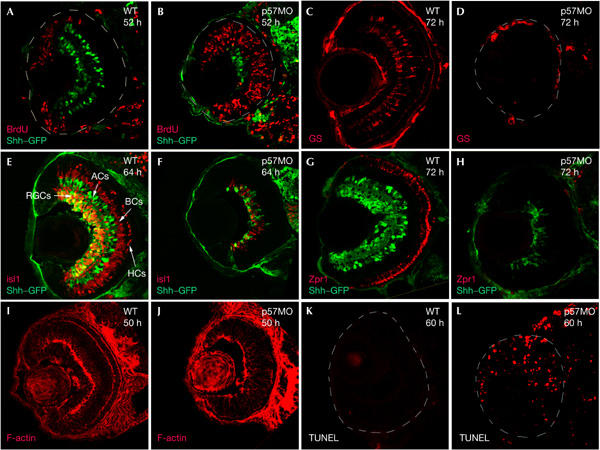
Targeted knockdown of p57Kip2 resembles the shh−/− retina. (A,B) Shh–GFP (green) and BrdU incorporation (red) in wild-type (A) and p57Kip2 knockdown embryos (B). BrdU incorporation (50–52 hpf). Note the similarity between (B) and Fig 1B. (C) Glutamine synthetase (GS) protein (red) is present in Mueller glia cells in the wild-type retina at 72 hpf, but is absent in p57Kip2 knockdown embryos (D). (E) Wild-type, at 64 hpf, Isl1 protein (red) and Shh–GFP (green). Isl1 is expressed in RGCs, amacrine, bipolar and horizontal cells. (F) The p57Kip2 knockdown retina shows overall reduction of Isl1-expressing and Shh–GFP-expressing cells at 64 hpf. (G) Zpr1 protein (red) is expressed in red–green double cones in the wild-type retina at 72 hpf, but is missing in the p57Kip2 knockdown (H). (I) F-actin staining (red) in the wild-type retina at 50 hpf stains the inner (IPL) and outer plexiform layers (OPL), but is absent in the p57Kip2 knockdown retina (J). (K,L) TUNEL assay performed on wild-type (K) and p57Kip2 knockdown retina (L) at 60 hpf shows an increase of apoptosis in p57Kip2 knockdown embryos. ACs, amacrine cells; BCs, bipolar cells; HCs, horizontal cells; MO, morpholino; RGCs, retinal ganglion cells.
p57Kip2 is sufficient to trigger cell-cycle exit
To investigate whether p57Kip2 is sufficient to trigger Shh-directed cell-cycle exit, we overexpressed p57Kip2 by using a bidirectional heat-shock (hs)-inducible promoter, which drives expression of both GFP and p57Kip2, thus allowing detection of p57Kip2-expressing cells by GFP expression (Bajoghli et al, 2004). Plasmid encoding the bidirectional hs-GFP/hs-p57Kip2 construct was injected into one-cell stage shh−/− embryos. In a control experiment, we injected plasmid encoding only hs-GFP. After activation of expression by heat shock at 24 hpf, we examined the effect of p57Kip2 on proliferation by BrdU labelling between 40 and 50 hpf. We found that in an shh mutant background, 83% of retinal cells expressing GFP alone were labelled with BrdU, but that only 18% of cells expressing both GFP and p57Kip2 were labelled with BrdU (Fig 4A–C), indicating that p57Kip2 is able to trigger cell-cycle exit in the retina. The fact that 18% of cells expressing p57Kip2 still incorporate BrdU might be because a subset of cells in the retina are not sensitive to p57Kip2, or because these cells were in a phase of the cell cycle that is not sensitive to p57Kip2. We also examined the effect of p57Kip2 on cell death in shh mutants with TUNEL labelling, and found that p57Kip2 suppresses apoptosis cell autonomously in the shh−/− retina (Fig 4D,E).
Figure 4.
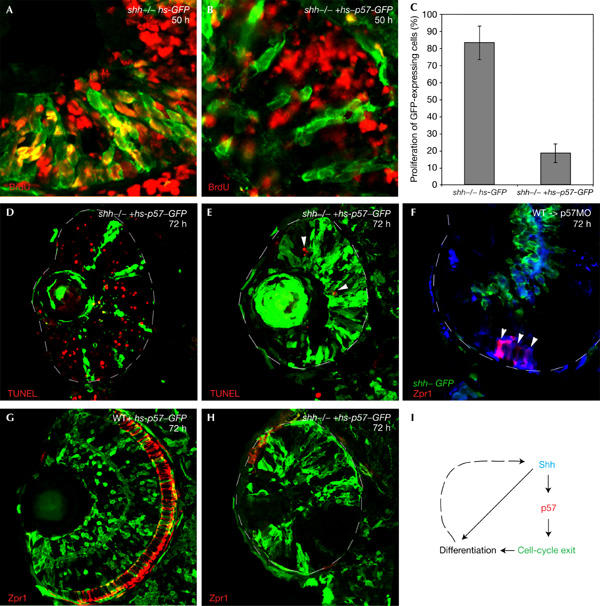
p57Kip2 rescues cell-cycle exit in shh mutants. (A) BrdU (40–50 hpf, red) in shh mutants expressing GFP (green) under the control of a heatshock promoter. Many GFP-expressing cells are also BrdU positive (yellow). (B) BrdU (40–50 hpf, red) in shh−/− embryos coexpressing p57Kip2 and GFP reporter (green) under the control of a bidirectional heat-shock promoter. None of the GFP-expressing cells is BrdU positive. (C) Graph summarizing the effect of p57Kip2 on proliferation. The proportion of BrdU-positive cells is markedly reduced by p57Kip2. (D,E) TUNEL assay on the shh−/− retina expressing p57Kip2/GFP at 72 hpf. Apoptosis is restricted to a few cells (arrowhead) that are not p57Kip2/GFP positive (E). (F) Wild-type cells transplanted into p57Kip2 knockdown embryos. Wild-type cells are labelled with biotin (blue) and are also transgenic for the Shh–GFP reporter (green). Zpr1-positive photoreceptors (red) were detected only in the wild-type cells (arrowheads). (G) Distribution of p57Kip2/GFP-expressing cells (green) in the wild-type retina at 72 hpf. Cells expressing p57Kip2/GFP are randomly distributed throughout the retina. (H) shh mutants with p57Kip2/GFP-expressing cells (green). Very few Zpr1-positive photoreceptors (red) are detectable. (I) Model for the role of p57Kip2 in mediating Shh-directed differentiation.
As knockdown of p57Kip2 leads to a strong reduction of shh expression in the retina (Fig 3A,B), it is possible that it functions upstream of shh activation. To address this question, we transplanted wild-type cells into p57Kip2 knockdown embryos, and asked whether they rescue nearby mutant retinal cells, as shown for shh (Shkumatava et al, 2004). We found that wild-type cells differentiate autonomously as photoreceptors, but failed to rescue nearby mutant cells (Fig 4F; data not shown), indicating that p57Kip2 activity is cell autonomously required for differentiation, and thus functions downstream of Shh, in cells that receive the Shh signal.
As p57Kip2 is able to trigger cell-cycle exit in shh mutants, this raises the question of whether exit from the cell cycle is sufficient to rescue differentiation in the absence of Shh. We failed to detect activation of marker genes of retinal differentiation, however, when we overexpressed p57Kip2 and GFP in shh mutants, and the p57Kip2-expressing cells retained the morphology of precursor cells under these conditions (Fig 4H; supplementary Fig 1H online). In wild-type embryos, p57Kip2-expressing cells are able to differentiate and contribute to all layers of the retina (Fig 4D; Supplementary Fig 1G online). Taken together, these results show that p57Kip2 is sufficient to trigger cell-cycle exit downstream of Shh signalling, but that cell-cycle exit alone is not sufficient for Shh-dependent differentiation in the retina.
Discussion
We have presented evidence here that Shh signalling is required for cell-cycle exit of precursor cells in the zebrafish retina, and that this effect is mediated by the CKI p57Kip2. Although transcriptional activation of p57Kip2 depends on Shh signalling in the retina, it is at present not clear whether this is a direct response to Shh or whether there are additional steps in between. Clearly, however, knockdown of p57Kip2 generates an shh mutant phenocopy: in the absence of either shh or p57Kip2 activity, precursor cells fail to exit the cell cycle and fail to differentiate, and instead continue to proliferate. Conversely, overexpression of p57Kip2 in the retina is sufficient to rescue cell-cycle exit in shh mutants. Taken together, these results indicate that p57Kip2 is an essential mediator of Shh signalling in the zebrafish retina, and is both necessary and sufficient for Shh-directed cell-cycle exit of precursor cells.
Zebrafish p57Kip2 is expressed much more broadly in the retina than in mouse p57Kip2, which is only expressed in about 16.5% of retinal cells (Dyer & Cepko, 2000). We found that zebrafish p57Kip2 is transiently expressed in the entire retinal epithelium, except for a patch of cells located in the ventronasal retina, which contains the first cells to become postmitotic. These cells differentiate independently of Shh signalling, and instead depend on a distinct signal originating from the optic stalk (reviewed by Neumann, 2001). Knockdown of p57Kip2 also does not affect differentiation of these cells, indicating that their cell-cycle exit must be controlled differently. Thus, p57Kip2 is only required for Shh-triggered cell-cycle exit, which sweeps as a wave through the retinal epithelium from its origin in the ventronasal retina. When precursor cells differentiate, they turn off p57Kip2, leading to transient expression in front of the advancing wave of differentiation. Some of the newly differentiated neurons then activate shh transcription, thereby propagating the wave further (Fig 4I).
Interestingly, there may be differences between the roles of Shh signalling in retinal differentiation in different vertebrate species. Thus, in the mouse, Shh seems to be required mainly for correct lamination of the retina (Wang et al, 2002), and in Xenopus, Hh signalling seems to have a role in differentiation of the pigmented retina (Perron et al, 2003).
Our previous results showed that Shh is required for differentiation of all principal cell types in the zebrafish retina (Shkumatava et al, 2004), and in the light of the data presented here, this suggests that the only function of Shh is to trigger cell-cycle exit of precursor cells in the retina. However, although p57Kip2 is sufficient to rescue cell-cycle exit in shh mutants, it is not sufficient to rescue differentiation in these mutants, indicating that in addition to triggering cell-cycle exit of precursor cells, Shh is also required for a distinct later step in differentiation (Fig 4F).
Knockdown of p57Kip2 does not equally affect all cell types, as we see a complete absence of photoreceptors and Mueller glia in knockdown embryos, whereas a small number of RGCs are still able to differentiate. Although this may be due to incomplete removal of p57Kip2 activity by our knockdown, an alternative possibility is that another CKI directs cell-cycle exit in these cells, similar to the situation in the mouse, where p27Kip1 and p57Kip2 control cell-cycle exit in distinct cell populations (Dyer & Cepko, 2001a). In contrast to the mouse, however, the relative contribution of the various CKIs is clearly different in the zebrafish retina, where p57Kip2 directs cell-cycle exit in most of the progenitor cells.
Taken together, our data indicate that the first step in Shh-dependent differentiation in the zebrafish retina is cell-cycle exit, and that Shh triggers this event through activation of p57Kip2. These findings raise the possibility that control of the core cell cycle may be part of a general mechanism whereby Hh signalling directs differentiation during development.
Methods
Zebrafish mutant lines. The shh allele syut4 and the Shh–GFP transgenic line 2.2Shh:gfp:ABC#15 were used as described by Shkumatava et al (2004).
BrdU labelling. BrdU labelling was performed as described by Link et al (2000).
TUNEL assay. Apoptotic cell death was detected by using In Situ Cell Death Detection kit (Roche, Indianapolis, IN, USA) according to the manufacturer's instructions.
Histochemical methods. In situ hybridization was performed on whole mounts, using p57Kip2, cyclin E2 and cyclin D1 as probes (Thisse et al, 2001). GenBank contains three zebrafish CKI sequences (p27a (AF398516), p27b (BI887574) and p57 (NM001002040)), but a sequence comparison indicates that p27b is a truncated form of zebrafish p57. Antibody labelling was perfomed on 12-μm-thick cryosections and analysed with a Leica confocal microscope. The following antibodies were used: rabbit anti-GFP (1:1,000; Torrey Pines Bioloabs, San Diego, CA, USA); mouse anti-Isl1 (1:50; Developmental Studies Hybridoma Bank, Iowa City, IA, USA); mouse anti-Zpr1 (1:200; University of Oregon, Eugene, OR, USA) and mouse anti-glutamine synthetase (1:500; BD Biosciences, San Jose, CA, USA). F-actin staining was carried out according to Shkumatava et al (2004), using Alexa Fluor 568-conjugated phalloidin (1:40; Molecular Probes, Eugene, OR, USA).
Morpholino injections. p57Kip2 antisense morpholino 5′-CCACGTTTGCCATGATGTCTAAAAG-3′ (Gene Tools, LLC, Philomath, OR, USA) in water was injected at the one-cell stage at 0.7 mM. As a control, we injected the standard control morpholino, available from Gene Tools, at the same concentration.
DNA constructs and microinjections. For p57Kip2 overexpression experiments, we generated the GFP:HSE:p57Kip2 construct, expressing both GFP and p57Kip2. We used the artificial heat-shock promoter (Bajoghli et al, 2004), driving bidirectional expression of both marker genes. Microinjections of plasmid DNA with meganuclease were carried out as described by Thermes et al (2002). The heatshock treatment was performed from 24 hpf as described by Bajoghli et al (2004).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figure 1
Acknowledgments
We thank G. Brunt and S. Fisher for excellent technical assistance, A. Stark and J. Stadler for scientific discussions and critical comments, T. Lunichenka for cloning of p57Kip2 and T. Czerny for providing the GFP:HSE construct.
References
- Bajoghli B, Aghaallaei N, Heimbucher T, Czerny T (2004) An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev Biol 271: 416–430 [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL (2000) p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development 127: 3593–3605 [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL (2001a) p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci 21: 4259–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL (2001b) Regulating proliferation during retinal development. Nat Rev Neurosci 2: 333–342 [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15: 3059–3087 [DOI] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH (2000) Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol 20: 9055–9067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BA, Fadool JM, Malicki J, Dowling JE (2000) The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells. Development 127: 2177–2188 [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL (2001) Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci 2: 109–118 [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC (2000) Effective targeted gene ‘knockdown' in zebrafish. Nat Genet 26: 216–220 [DOI] [PubMed] [Google Scholar]
- Neumann CJ (2001) Pattern formation in the zebrafish retina. Semin Cell Dev Biol 12: 485–490 [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Nuesslein-Volhard C (2000) Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science 289: 2137–2139 [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA (2003) Neurogenesis and the cell cycle. Neuron 40: 199–208 [DOI] [PubMed] [Google Scholar]
- Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Piler T, Harris WA (2003) A novel function for Hedgehog signaling in retinal pigmented epithelium differentiation. Development 130: 1565–1577 [DOI] [PubMed] [Google Scholar]
- Roy S, Ingham PW (2002) Hedgehogs tryst with the cell cycle. J Cell Sci 115: 4393–4397 [DOI] [PubMed] [Google Scholar]
- Shkumatava A, Fischer S, Muller F, Strahle U, Neumann CJ (2004) Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development 131: 3849–3858 [DOI] [PubMed] [Google Scholar]
- Stadler J, Shkumatava A, Neumann CJ (2004) The role of Hedgehog signaling in the development of the zebrafish visual system. Dev Neurosci 26: 346–351 [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA, Mallory DE, Shupe EE (2002) Embryonic retinal gene expression in sonic-you mutant zebrafish. Dev Dyn 225: 344–350 [DOI] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS (2002) IsceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118: 91–98 [DOI] [PubMed] [Google Scholar]
- Thisse B et al. (2001) Expression of the zebrafish genome during embryogenesis (NIH R01 RR15402). ZFIN Direct Data Submission (www.zfin.org)
- Wang YP, Dakubo G, Howley P, Campsall KD, Mazarolle CJ, Shiga SA, Lewis PM, McMahon AP, Wallace VA (2002) Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat Neurosci 5: 831–832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1


