Abstract
Targeted expression of biologically active interleukin-12 (IL-12) in astrocytes of the central nervous system (CNS) results in spontaneous neuroimmunological disease of aged mice. Borna disease virus (BDV) can readily multiply in the mouse CNS but does not trigger disease in most strains. Here we show that a large percentage of IL-12 transgenic mice developed severe ataxia within 5 to 10 weeks after infection with BDV. By contrast, no disease developed in mock-infected IL-12 transgenic and wild-type mice until 4 months of age. Neurological symptoms were rare in infected wild-type animals, and if they occurred, these were milder and appeared later. Histological analyses showed that the cerebellum of infected IL-12 transgenic mice, which is the brain region with strongest transgene expression, contained large numbers of CD4+ and CD8+ T cells as well as lower numbers of B cells, whereas other parts of the CNS showed only mild infiltration by lymphocytes. The cerebellum of diseased mice further showed severe astrogliosis, calcifications and signs of neurodegeneration. BDV antigen and nucleic acids were present in lower amounts in the inflamed cerebellum of infected transgenic mice than in the noninflamed cerebellum of infected wild-type littermates, suggesting that IL-12 or IL-12-induced cytokines exhibited antiviral activity. We propose that BDV infection accelerates the frequency by which immune cells such as lymphocytes and NK cells enter the CNS and then respond to IL-12 present in the local milieu causing disease. Our results illustrate that infection of the CNS with a virus that is benign in certain hosts can be harmful in such normally disease-resistant hosts if the tissue is unfavorably preconditioned by proinflammatory cytokines.
Interleukin-12 (IL-12) is a key regulator of cellular immune responses (11, 45). This heterodimeric cytokine acts on T cells, NK cells and B cells. In T cells, IL-12 induces T-cell differentiation from the Th0 to the Th1 state. Upon IL-12 stimulation, T and NK cells produce gamma interferon (IFN-γ) and proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-1. Due to these effects, IL-12 is a critical mediator in the immune response against infectious agents such as parasites, viruses, and bacteria as well as against tumors (reviewed in references 6 and 45). IL-12 plays a crucial role in the development of autoimmune diseases such as experimental allergic encephalomyelitis (EAE), an animal model for the human disorder multiple sclerosis (MS) (20, 39, 47). Treatment of mice with IL-12 antibodies ameliorates the clinical course of the neurological disorder in EAE (20, 47), and mice bearing a targeted disruption of the IL-12 gene are resistant to EAE (39). The observed cerebral expression of IL-12 in EAE (7, 16) as well as in MS (3, 48) and in the course of lipopolysaccharide-induced endotoxemia (33, 43) implies that this cytokine has the potential to alter the immune state of the central nervous system (CNS) under a variety of pathological conditions.
We recently developed transgenic mice (GF-IL-12 mice) that express IL-12 specifically in astrocytes under the transcriptional control of the glial fibrillary acidic protein (GFAP) promoter (31). At around 4 to 6 months of age, a fraction of these mice spontaneously develop a neuroinflammatory disorder that is characterized by severe mononuclear meningoencephalitis and calcifications in the cerebellum, the CNS region with maximum transgene expression. Immunization of presymptomatic GF-IL-12 mice with complete Freund's adjuvant (CFA) without addition of CNS antigen triggers a similar Th1-like immune response in the CNS of most GF-IL-12 mice (19). Continuous cerebral expression of IL-12 thus seems to generate a proinflammatory immune milieu within the CNS that promotes the infiltration and stimulation of peripherally activated T and NK cells.
To determine how the IL-12-sensitized CNS would cope with a viral infection, we examined the consequences of neonatal infection of GF-IL-12 mice with Borna disease virus (BDV). BDV is a nonsegmented, negative-stranded RNA virus that can persistently infect the CNS of a variety of animal species (for a review, see reference 41), which may then develop a severe nonpurulent meningoencephalitis (12, 21, 37). Neurological disease involves a cellular immune response that depends on both CD4+ and CD8+ T cells (4, 28, 35, 36, 44; O. Planz, C. Rentzsch, A. Batra, H. J. Rziha, and L. Stitz, Letter, Lancet 352:623, 1998). Although BDV can replicate in the mouse CNS, most inbred mouse strains, including the one used in this study, show a high degree of resistance to BDV-induced neurological disorder. Here we show that BDV infection triggered early onset of disease in GF-IL-12 mice.
MATERIALS AND METHODS
Animals.
The generation and characterization of GF-IL-12 mice expressing both subunits of IL-12 under transcriptional control of the astrocyte-specific GFAP promoter have been described previously (31). The mice were on a C57BL/6 × SJL hybrid background and heterozygous for the IL-12 transgene, providing heterozygous transgenic and wild-type offspring in the same litter. At an age of 2 weeks, transgenic offspring were identified by PCR analysis of tail DNA as described previously (31). Breeding was performed in the animal facility of the University of Freiburg.
Virus stocks and infection of mice.
A rat-adapted strain of BDV was adapted to the mouse by four consecutive passages through brains of newborn BALB/c mice and two further passages through brains of adult MRL mice. The seed virus which was originally assumed to be derived from strain He/80 (13) has recently been identified as strain “rat BDV,” also designated RW98 (10). Intracerebral infection of newborn mice (less than 48 h after birth) was performed by injecting 10-μl samples of 10% brain homogenate (stock 76) into the left brain hemispheres using a Hamilton syringe. Mock infections were performed with 10 μl of a 10% brain homogenate derived from uninfected BALB/c mice. Mice were examined daily for symptoms of neurological disease such as ataxia, ruffled fur, and reduced activity until 120 days after infection.
RNA isolation.
Brains were removed and one hemisphere was dissected into forebrain and cerebellum, the other hemisphere was processed for histological examination (see below). The dissected samples were immediately snap-frozen in liquid nitrogen and stored at −80°C pending RNA extraction. Total RNA was extracted with Trizol reagent (Gibco BRL, Grand Island, NY) according to the manufacturer's protocol. The RNA was dissolved in TE (10 mM Tris [pH 8], 1 mM EDTA) and stored at −80°C.
RNase protection assays.
RNase protection assays for the detection of cytokine RNAs were performed as described previously (42). The RNA samples were hybridized with labeled probe sets IC5 (8); AP8, which contains probes for the IL-12 p40-hGH and p35-hGH transgene (31); probe sets for the detection of chemokines (CK1) (1) and IFN-α and IFN-β (2); or a novel probe set (AP9) containing probes for CD3, BDVp40 (38), NOS-1, NOS-2, and NOS-3 as well as TNF-α, IFN-γ, IL-1α, IL-10, IL-12, and GFAP. In all probe sets, a fragment of the RPL32-4A gene (9) served as an internal loading control. NIH Image software, version 1.62, was used to quantify the autoradiographs.
Histology and IHC.
For routine histology, the brain hemispheres were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Sections (4 μm thick) were processed according to standard procedures and used for routine staining and immunohistochemistry (IHC) for GFAP (DAKO, Hamburg, Germany), BDV p40 (kind gift from I. Lipkin, University of California—Irvine), and signal transducer and activator of transcription 1 (STAT1) (Santa Cruz Biotechnology, Heidelberg, Germany). Alizarin Red stain was used for the detection of calcifications. For immunophenotyping and cellular adhesion molecule immunostaining, the hemispheres were immediately embedded in Tissue Tek (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands), snap-frozen in liquid nitrogen-chilled isopentane, and stored at −80°C. Sagittal cryomicrotome sections 10 μm thick were air-dried and fixed in cold (−20°C) acetone-methanol (1:1) for 45 s. Subsequently, the sections were incubated in normal serum for 30 min (ABC kit; Vector Laboratories, Burlingame, Calif.). Sections were then incubated overnight at 4°C with rat monoclonal antibodies to identify markers of infiltrating lymphocytes (CD4, CD8, and B220), activation markers (B7-1, B7-2, and Mac-3), and cellular adhesion molecules (intercellular adhesion molecule 1 [ICAM-1] and vascular cell adhesion molecule) (all from PharMingen, Hamburg, Germany). Bound antibody was detected using a mouse-absorbed, biotinylated anti-rat antibody (Southern Biotechnology Associates, Birmingham, Ala.) followed by avidin-labeled horseradish peroxidase (Sigma, Deisenhofen, Germany) and 3-3-diaminobenzidine (Sigma) as a substrate. Sections were counterstained with Mayer's hematoxylin, dehydrated in graded ethanols, and mounted.
Flow cytometry analysis of brain lymphocytes.
Lymphocytes from severely diseased animals were isolated essentially as described previously (15, 34). Approximately 5 × 105 brain lymphocytes were resuspended in phosphate-buffered saline containing 2% fetal calf serum, 0.1% NaN3, and heparin (Liquemin [10 U/ml]; Hoffmann-LaRoche, Basel, Switzerland). The cell suspension was incubated with R-phycoerythrin-labeled anti-CD4 and fluorescein-labeled anti-CD8 antibodies (1:200; Life Technologies, Eggenstein, Germany) for 20 min at room temperature. After washing, analysis of cells was performed on a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany).
RESULTS
BDV accelerates cerebellar neuroinflammation and disease in GF-IL-12 mice.
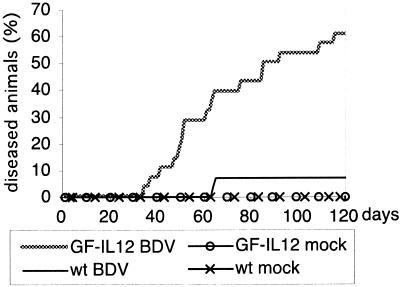
BDV-infected or mock-infected GF-IL-12 mice and their wild-type littermates were examined daily for the presence of neurological disease up to an age of 120 days. Beginning at day 35 postinfection (p.i.), an increasing number of infected GF-IL-12 mice developed progressive clinical symptoms, including a nonphysiological position of the hind limbs when lifted by the tail, severe ataxia, hunched posture, and ruffled fur. Over time, 17 out of 28 GF-IL-12 mice (61%) presented with neurological disorder, while only 2 out of the 28 wild-type littermates (7%) became sick (Fig. 1). No sick mouse recovered from disease. The onset of disease in wild-type mice was delayed, and disease symptoms were milder than those exhibited by GF-IL-12 animals. Mock-infected wild-type and GF-IL-12 mice (six each) showed no clinical symptoms during the entire investigation period. This finding is consistent with previous observations that the spontaneously occurring disorder in GF-IL-12 mice does not develop before 4 months of age (31). All infected mice had BDV antigen in the CNS (see below).
FIG. 1.
Neurological disorder in BDV-infected mice. Newborn GF-IL-12 and wild-type (wt) mice were infected intracerebrally with BDV as described in Materials and Methods. Mice were examined daily for clinical symptoms of neurologic disease. Note that mock-infected mice of neither strain developed neurological symptoms.
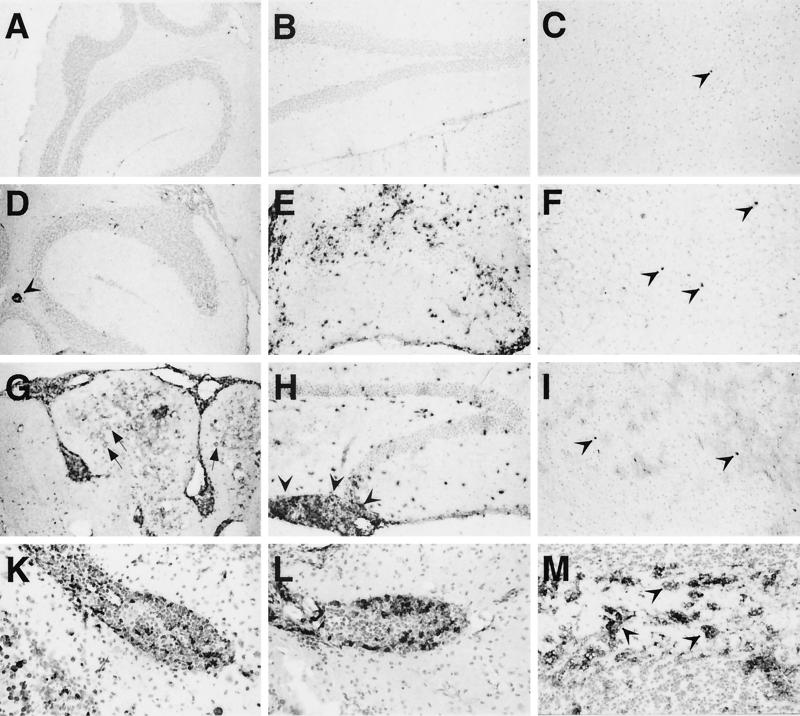
Histological examination of the brains of healthy BDV-infected wild-type mice revealed no overt histological alterations and there were no infiltrating lymphocytes observed (Fig. 2A to C). Some of the healthy BDV-infected GF-IL-12 mice had few mononuclear infiltrations in the CNS (data not shown). In most forebrain areas and in the cerebellum of the two sick wild-type mice there was scant infiltration of mononuclear cells (Fig. 2D and F). This contrasted with dense lymphocytic infiltration in the hippocampus (Fig. 2E). While comparably scant infiltration was observed in the thalamus of sick GF-IL-12 mice (Fig. 2I), there was less-pronounced infiltration in the hippocampus but severe infiltration of the pia mater between the hippocampus and the thalamus (Fig. 2H). Progressive mononuclear infiltration of the cerebellar parenchyma and of the meninges as well as perivascular cuffing in the cerebellum, the dominant site of transgenic IL-12 expression, was observed in mice beginning around 6 weeks of age (Fig. 2G). Additional features observed were severe neurodegeneration with loss of granule cells and calcification of the granule cell layer in the cerebellum of sick GF-IL-12 mice. IHC analysis revealed that the infiltrating mononuclear cells were predominantly CD4+ and to a lesser extent CD8+ T cells as well as CD45R+ B cells and foamy macrophages (Fig. 2K to M). Isolation of brain lymphocytes from diseased GF-IL-12 mice and subsequent analysis of T-cell subsets by flow cytometry revealed that the CD8/CD4 ratio in the brains was 0.7 (n = 8). This contrasts with the findings in CNS infiltrates of MRL mice that develop spontaneous disease after infection with BDV, where the CD8/CD4 ratio is around 2 in acute disease (14). There was also upregulation of ICAM-1, B7-2, and major histocompatibility complex class II (data not shown). These alterations were moderate in all parts of the CNS of sick wild-type mice and in the forebrain of sick GF-IL-12 mice, but very pronounced in the cerebellum of sick GF-IL-12 animals. Mock-infected mice of both lines showed neither infiltration by mononuclear cells nor upregulation of cell adhesion molecules at 120 days p.i. when the experiment was terminated. Thus, BDV was well tolerated by wild-type mice, while it greatly accelerated cerebellar inflammation and neurodegeneration in GF-IL-12 mice.
FIG. 2.
Pathological alterations in brains of BDV-infected wild-type and GF-IL-12 mice. IHC was performed as described in Materials and Methods. Cerebellum (A), hippocampus (B), and thalamus (C) specimens of healthy BDV-infected wild-type mice showed no CD4-immunoreactive T cells in the parenchyma. (C) Immunoreactivity for CD4 of an intravascular lymphocyte is indicated (arrowhead). Sick wild-type mice (D to F) showed dense infiltration of CD4 T cells in the hippocampus (E) and scant infiltration in the cerebellum and thalamus (D and F) (arrowheads). (G) In GF-IL-12 mice, severe neurodegeneration with loss of granule cells, mononuclear meningoencephalitis, marked astrocytosis, and parenchymal calcifications (arrows) of the cerebellum were observed. There were abundant CD4 T cells in the pia mater between hippocampus and thalamus (H) (arrowheads), while hippocampal infiltration was less severe than that observed for sick wild-type mice (E). Lymphocytic infiltration of the thalamus (I) (arrowheads) was comparable to that seen in the thalamus of sick wild-type mice. (A to I) (IHC for CD4). Phenotyping of infiltrating mononuclear cells in GF-IL-12 mice revealed CD8 T cells (K) as well as CD45R+ B cells (L) and Mac-3 immunoreactive foamy macrophages (M) (arrowheads).
BDV-triggered synthesis of chemokines, cytokines, and STAT-1 in GF-IL-12 mice.
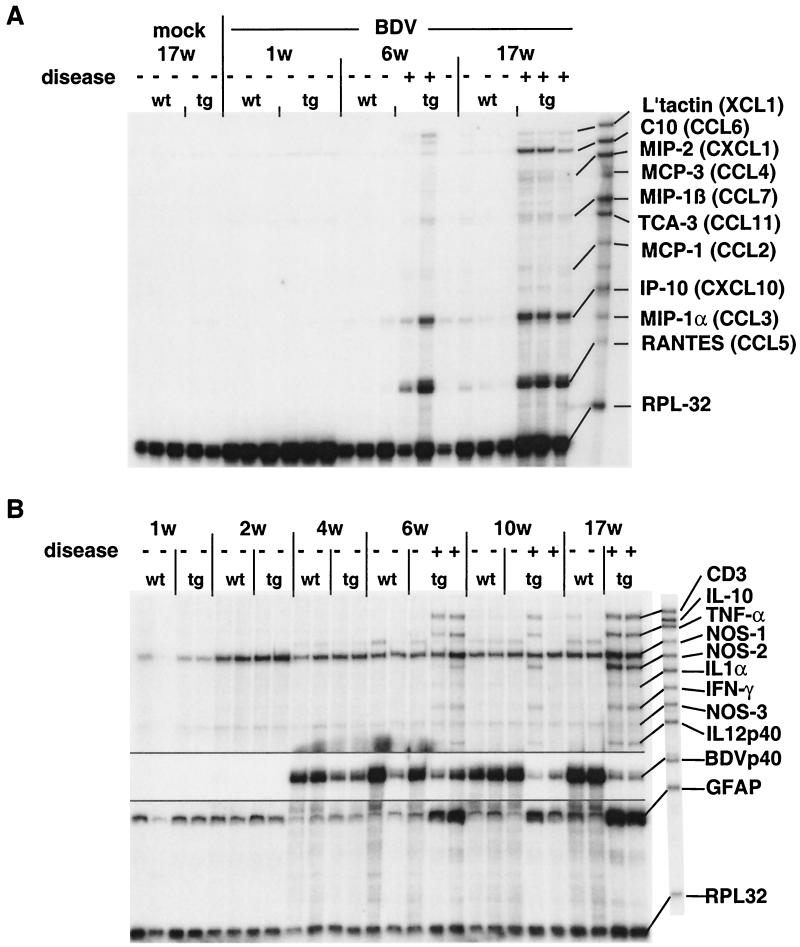
To further characterize the neuroinflammatory process in the cerebellum of GF-IL-12 mice, we determined the expression of a number of genes for chemokines, cytokines, cell surface molecules, and nitric oxide synthetase isoforms. Constitutive low-level expression of CCL6 (C10) chemokine RNA was observed in the hindbrain (Fig. 3A). Starting around 6 weeks of age we observed expression of the CXCL10 (IP-10) and CCL5 (RANTES) genes in the hindbrain of both BDV-infected wild-type and GF-IL-12 mice. However, GF-IL-12 mice showed enhanced expression of these genes. In addition, we observed enhanced levels of XCL1 (lymphotactin), CCL6, CXCL1 (MIP-2), CCL4 (MIP-1β), and CCL2 (MCP-1) transcripts in the hindbrain of BDV-infected GF-IL-12 mice more than 6 weeks of age (Fig. 3A). In the forebrain, both GF-IL-12 and wild-type mice showed comparable low levels of constitutive TNF-α and high levels of TGF-β RNA (data not shown). All but one sick GF-IL-12 mouse (Fig. 3B, 10 weeks, lane 5) older than 6 weeks had high levels of IFN-γ, TNF-α, IL-1α and NOS-2 transcripts in the cerebellum that were associated with the presence of CD3 transcripts (Fig. 3B). By contrast, starting at 6 weeks of age only 2 of the 22 wild-type mice showed low-level expression of TNF-α and IL-1α in the cerebellum (data not shown). Expression of IFN-α or IFN-β genes was not detectable by RNase protection assay in the CNS of wild-type and GF-IL-12 mice (data not shown). Consistent with the scant infiltration by T cells observed by IHC at 10 and 17 weeks, there were low levels of CD3 RNA in the cerebellum of some infected wild-type mice. Moreover, we observed a steadily increasing expression level of ICAM-1, A20, MAC-1, and EB22/5 in the cerebellum of sick wild-type and GF-IL-12 mice (data not shown).
FIG. 3.
Expression of various cytokine and chemokine genes in the cerebellum of BDV-infected mice. (A) RNase protection assay with the chemokine probe set CK1. L'tactin, lymphotactin. (B) RNase protection assay with the probe set AP9. In order to optimize the visualization of the different bands, the same gel was exposed to autoradiographic film for various periods of time. The upper part of the gel was exposed overnight, the intermediate (BDVp40) portion was exposed for 4 h, and the lower portion was exposed for 6 h.
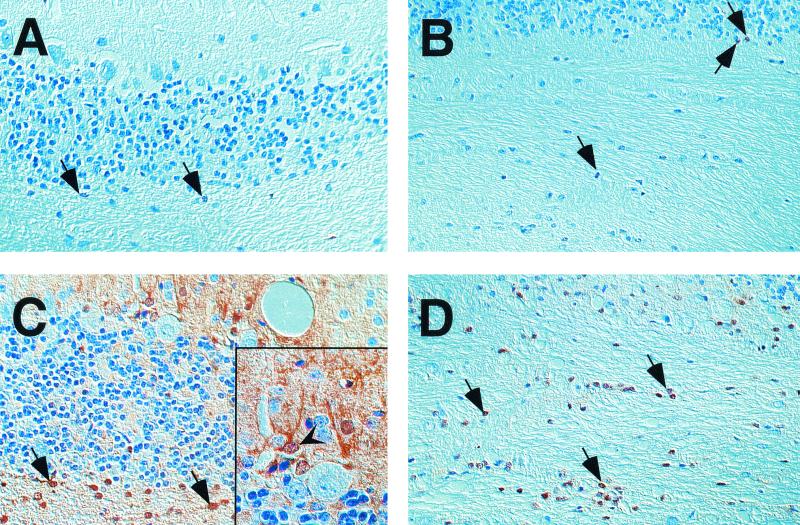
In GF-IL-12 mice that develop spontaneous disease at older age, high levels of STAT-1 signaling molecules were previously observed (22). We therefore determined whether this was also true for GF-IL-12 mice with BDV-triggered early disease onset. In the forebrain of infected, sick GF-IL-12 mice only slight increases in the number of STAT-1 immunoreactive cells (data not shown) were observed, whereas strong STAT-1 labeling was evident in the cerebellum (Fig. 4 C and D). The abundant nuclear localization of the STAT-1 molecule was consistent with active IFN-γ signaling. In the forebrain (data not shown) and in the cerebellum (Fig. 4A and B) of BDV-infected, asymptomatic wild-type mice, only few STAT-1 immunoreactive cells were present.
FIG. 4.
IHC for STAT1 revealed few immunoreactive cells in the cerebellar cortex (A) and white matter (B) of infected wild-type mice. In the respective brain regions of GF-IL-12 mice, strong expression of STAT1 was found (in the cortex [C] and the white matter [D]). Nuclear localization of STAT1 immunoreactivity in the cerebellar white matter (arrows). In infected GF-IL-12 mice there is additional immunoreactivity in the nuclei and the cytoplasm of cells resembling basket neurons (arrowhead). Magnification, ×40; magnification of inset, ×100.
Reduced replication of BDV in the cerebellum of GF-IL-12 mice.
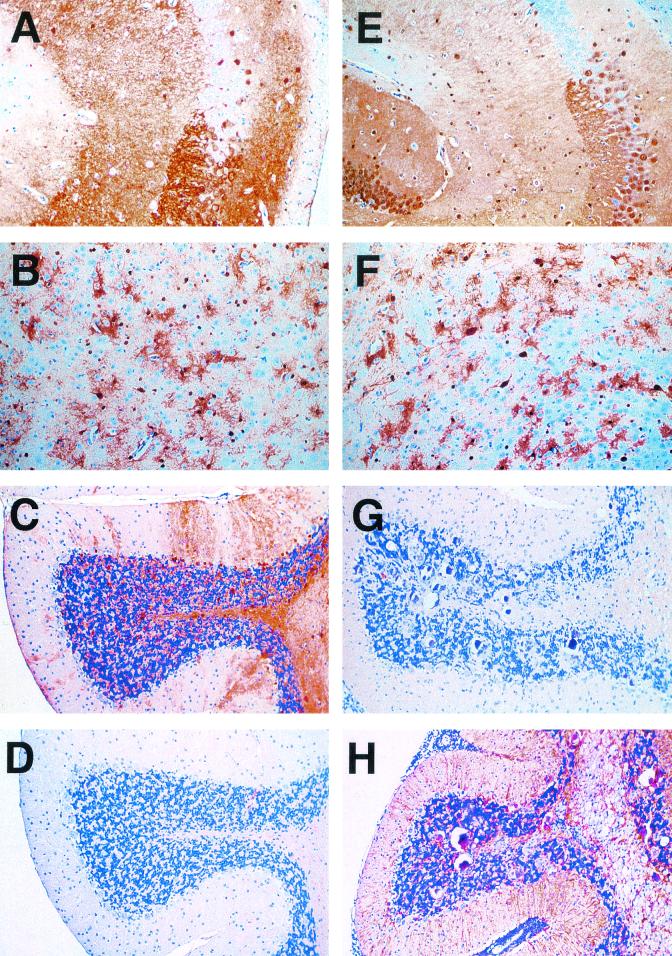
IHC staining for the p40 nucleoprotein of BDV in the forebrain of infected wild-type (Fig. 5A and B) and GF-IL-12 mice (Fig. 5E and F) revealed comparable antigen levels in both groups. In the cerebellum of the majority of GF-IL-12 mice, however, immunoreactivity for BDVp40 was significantly reduced (Fig. 5G) compared to wild-type mice (Fig. 5C). IHC examination further revealed moderate upregulation of GFAP immunoreactivity in the cerebellum of infected wild-type mice (Fig. 5D), which contrasted the pronounced upregulation of GFAP in the cerebellum of sick GF-IL-12 mice (Fig. 5H). GFAP immunostaining was weak in the forebrain of both wild-type and GF-IL-12 mice (data not shown).
FIG. 5.
Reduced viral antigen levels in the cerebellum of GF-IL-12 mice. IHC staining for BDVp40 revealed virtually identical patterns in hippocampus (A and E) and thalamus (B and F) specimens from wild-type (A to D) and GF-IL-12 (E to H) mice. In contrast, in the cerebellum of GF-IL-12 mice (G), BDVp40 immunoreactivity was strongly reduced compared with that observed for wild-type mice (C). An inverse staining pattern was observed for GFAP. Immunoreactivity for GFAP was stronger in the cerebellum of GF-IL-12 mice (H) than in that of wild-type mice (D).
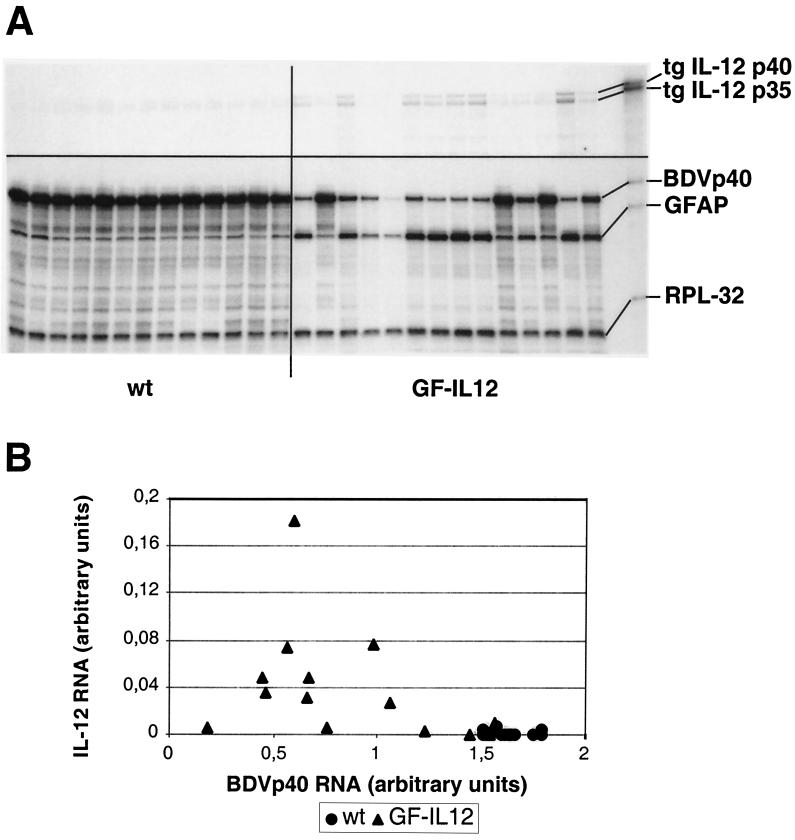
To investigate whether the observed differences in viral protein levels correlated with RNA levels, RNase protection assays were performed. This analysis revealed high levels of BDVp40 RNA expression by 4 weeks of age that did not change significantly at later time points (Fig. 3B and data not shown). Importantly, we observed significantly lower levels of BDVp40 RNA and increased levels of GFAP RNA in the cerebellum of GF-IL-12 mice compared to the wild-type littermates (Fig. 6A). This difference was dependent on the presence of IL-12 transgene expression, since most GF-IL-12 mice with very low transgene expression showed cerebellar GFAP and BDVp40 gene expression comparable to wild-type mice (P ≤ 0.002, Mann-Whitney test; Fig. 6B).
FIG. 6.
Reduced BDV transcript levels in the cerebellum specimens of GF-IL-12 mice. (A) RNase protection assays were performed as described in Materials and Methods. Note the very low levels of transgene (tg) expression in the cerebellum specimens of five GF-IL-12 mice. (B) Quantification of IL-12 and BDVp40 gene expression in the cerebellum of wild-type (wt) and GF-IL-12 mice that expressed the transgene revealed highly significant differences (P ≤ 0.002 [Mann-Whitney]).
DISCUSSION
We previously showed that around the ages of 4 to 6 months, GF-IL-12 mice spontaneously develop a mononuclear meningoencephalitis that is accompanied by severe astrocytosis and tissue destruction in the cerebellum (31). Active immunization of GF-IL-12 mice with CFA, pertussis toxin and myelin antigen led to earlier onset of EAE compared to immunization of wild-type mice. Similarly, immunization of GF-IL-12 mice with CFA and pertussis toxin without CNS-specific antigen induced disease symptoms that resembled the otherwise spontaneously developing disorder in GF-IL-12 mice (19). Our present data demonstrate that infection of the CNS with a noncytolytic virus that is largely ignored by the host immune system can trigger a cascade of events that greatly accelerates disease progression in GF-IL-12 mice. In the experiments presented here, 61% of GF-IL-12 mice became sick by 120 days p.i. This time point was chosen because at later time points we would not be able to distinguish between spontaneous and BDV-induced neurological disorder in GF-IL-12 mice. The onset of neurological symptoms seems to be influenced by the genetic background of the mice since 100% of BDV-infected GF-IL-12 mice on a pure C57BL/6 background developed disease by 60 days p.i. (data not shown). This observation suggests that at later time points all BDV-infected GF-IL-12 mice on the SJL × C57BL/6 background would develop neurological disorder. The broad range of IL-12 RNA expression levels in the cerebellum of BDV-infected GF-IL-12 mice (Fig. 6A) might be explained by numerous factors such as genetic background as well as number and activation of infiltrating mononuclear cells that could act on the GFAP-promoter in an inflamed tissue thereby enhancing transgene-encoded IL-12 expression.
We and others have shown previously that a number of chemokine genes are strongly expressed in the BDV-infected CNS and may trigger lymphocyte infiltration in immunocompetent animals (24, 38). We propose that BDV accelerates disease in GF-IL-12 mice by increasing the frequency by which immune cells such as lymphocytes and NK cells are entering the CNS. In the cerebellum of GF-IL-12 mice, these cells presumably meet a milieu that allows them to survive and proliferate. In mock-infected GF-IL-12 mice, lymphocytic traffic is not stimulated and disease onset is delayed. It is not clear at present whether or not the disease-inducing immune response in the CNS of BDV-infected GF-IL-12 mice was directed against viral antigens. If it was CTL-mediated, we would expect to find CD8 T cells in all brain areas that carry virus, which was clearly not the case. In agreement with this notion, mononuclear infiltrations were found mainly in the hind brain of BDV-infected GF-IL-12 mice, whereas they were present in all brain regions of MRL mice that develop neurological disease spontaneously after infection with BDV (14). Furthermore, the CD4 T cells dominated in brains of diseased GF-IL-12 mice. The ratio of CD8 to CD4 T cells in the brains of these mice was 0.7, whereas this ratio was about 2 for cells from the brains of BDV-infected MRL mice (14). The results of a preliminary study indicate that CD8 T cells from brains of BDV-infected GF-IL-12 mice cannot lyse target cells expressing the BDV nucleoprotein which is the major target of the CTL response in MRL and other H-2k mice (J. Hausmann and A. Pagenstecher, unpublished data). At present we cannot exclude the possibility that the number of BDVp40-reactive T cells was below the detection limit of the technique used. Alternatively, another BDV protein may represent the major CTL target in H-2b/H-2s mice that were used in this study. Finally, it is also possible that IL-12 stimulated the development of viral-specific CD4 T cells that then might mediate the observed antiviral response. Future experiments by us will examine these possibilities further.
How can T cells survive and proliferate in the CNS of GF-IL-12 mice if they lack detectable specificity for viral or self antigens? Recent evidence suggests that CD4 T cells can be activated to secrete IFN-γ in the absence of antigen if their IL-12 and IL-18 receptors are triggered simultaneously (25). Unlike the classical acquired activation pathway that is dependent on signals from the antigen-activated T-cell receptor, the innate activation pathway is antigen-independent but dependent on IL-12 and IL-18, which together activate the p38 mitogen-activated protein kinase in CD4 T cells (49). In our transgenic mice, IL-12 is present in the cerebellum, raising the question regarding the source of IL-18, which might act in concert. The IL-18 gene is expressed constitutively and inactive cytokine precursors are permanently present in many cell types, including microglia and astrocytes (26), which may be cleaved and released in response to as yet undefined stimuli in our mice. The microenvironment in the cerebellum generated by the simultaneous presence of IL-12 and IL-18 is probably of crucial importance for the development of the type of neuroinflammatory disease that we observe in GF-IL-12 mice.
The observed reduction of BDV-derived nucleic acid and protein in the hindbrain of GF-IL-12 mice is likely caused by IL-12 or by IL-12 inducible factors. IL-12 is a potent inducer of cytokines which can mediate antiviral effects including IFN-γ and TNF-α (for a review, see reference 6). In the case of murine cytomegalovirus, the antiviral property of IL-12 was attributed to early synthesis of IFN-γ by NK cells (29, 30). Recently, Binder and Griffin demonstrated that IFN-γ mediates site-specific clearance of an alphavirus from neurons (5). Similarly, in passively immunized mice deficient for T and B cells, IL-12-induced IFN-γ from NK cells was sufficient for the control of herpes simplex virus (46). In contrast, IL-12-induced inhibition of vesicular stomatitis virus in the CNS required neither TNF-α nor IFN-γ but was dependent on NOS-1 (17, 18). A recent study extended these findings by demonstrating that IFN-γ-inducible NOS-2 is crucial for eliminating murine cytomegalovirus (27). In the cerebellum of BDV-infected GF-IL-12 mice, we observed induction of TNF-α, IFN-γ, and IL-1 as well as NOS-2 genes, while NOS-1 and NOS-3 transcripts remained at control levels. The putative soluble factors that are involved in this antiviral effect remain to be identified.
Our results demonstrate for the first time that a nonsymptomatic viral infection of the CNS may take a devastating course if there is cerebral production of the proinflammatory cytokine IL-12. Thus, infection of the CNS with a virus that is benign in certain hosts can be harmful in such normally disease-resistant hosts if the tissue is unfavorably preconditioned by proinflammatory cytokines. In our model system, IL-12 was supplied by expression of appropriate transgenes in astrocytes. However, cerebral expression of IL-12 has been observed in the human demyelinating disease MS (3, 48) and its animal model, EAE (7, 16), as well as in the course of lipopolysaccharide-induced endotoxemia (33, 43). Interestingly, antecedent viral and bacterial infections are associated with the development or exacerbation of MS (23, 32, 40). Our observations suggest that IL-12 found in such circumstances could target and fuel the immune response initiated by a viral infection, causing more severe immune pathology and disease severity in the CNS.
Acknowledgments
We thank Eva M. Wussler and Barbara Herbstritt for their excellent technical assistance, M. Olschewski for help with the statistical evaluation, and O. Haller for critical reading of the manuscript.
This work was supported by grants of the Deutsche Forschungsgemeinschaft and ZKF I Freiburg to A.P. and NIH grant NS 36979 to I.L.C.
REFERENCES
- 1.Asensio, V. C., and I. L. Campbell. 1997. Chemokine gene expression in the brain in lymphocytic choriomeningitis. J. Virol. 71:7832-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asensio, V. C., C. Kincaid, and I. L. Campbell. 1999. Chemokines and the inflammatory response to viral infection in the central nervous system with a focus on lymphocytic choriomeningitis virus. J. Neurovirol. 5:65-75. [DOI] [PubMed] [Google Scholar]
- 3.Balashov, K. E., D. R. Smith, S. J. Khoury, D. A. Hafler, and H. L. Weiner. 1997. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc. Natl. Acad. Sci. USA 94:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilzer, T., and L. Stitz. 1994. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J. Immunol. 153:818-823. [PubMed] [Google Scholar]
- 5.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 7.Bright, J. J., B. F. Musuro, C. Du, and S. Sriram. 1998. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J. Neuroimmunol. 82:22-30. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, I. L., M. Eddleston, P. Kemper, M. B. Oldstone, and M. V. Hobbs. 1994. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J. Virol. 68:2383-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudov, K. P., and R. P. Perry. 1984. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 37:457-464. [DOI] [PubMed] [Google Scholar]
- 10.Formella, S., C. Jehle, C. Sauder, P. Staeheli, and M. Schwemmle. 2000. Sequence variability of Borna disease virus: resistance to superinfection may contribute to high genome stability in persistently infected cells. J. Virol. 74:7878-7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-5211. [DOI] [PubMed] [Google Scholar]
- 12.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 13.Hallensleben, W., M. Schwemmle, J. Hausmann, L. Stitz, B. Volk, A. Pagenstecher, and P. Staeheli. 1998. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J. Virol. 72:4379-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausmann, J., W. Hallensleben, J. C. de la Torre, A. Pagenstecher, C. Zimmermann, H. Pircher, and P. Staeheli. 1999. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 96:9769-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani, D. N., and D. E. Griffin. 1991. Isolation of brain parenchymal lymphocytes for flow cytometric analysis. Application to acute viral encephalitis. J. Immunol. Methods 139:223-231. [DOI] [PubMed] [Google Scholar]
- 16.Issazadeh, S., A. Ljungdahl, B. Hojeberg, M. Mustafa, and T. Olsson. 1995. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J. Neuroimmunol. 61:205-212. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu, T., D. D. Ireland, N. Chen, and C. S. Reiss. 1999. Neuronal expression of NOS-1 is required for host recovery from viral encephalitis. Virology 258:389-395. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu, T., and C. S. Reiss. 1997. IFN-gamma is not required in the IL-12 response to vesicular stomatitis virus infection of the olfactory bulb. J. Immunol. 159:3444-3452. [PubMed] [Google Scholar]
- 19.Lassmann, S., C. Kincaid, V. C. Asensio, and I. L. Campbell. 2001. Induction of type 1 immune pathology in the brain following immunization without central nervous system autoantigen in transgenic mice with astrocyte-targeted expression of IL-12. J. Immunol. 167:5485-5493. [DOI] [PubMed] [Google Scholar]
- 20.Leonard, J. P., K. E. Waldburger, and S. J. Goldman. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 181:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 22.Maier, J., C. Kincaid, A. Pagenstecher, and I. L. Campbell. 2002. Regulation of signal transducer and activator of transcription and suppressor of cytokine-signaling gene expression in the brain of mice with astrocyte-targeted production of interleukin-12 or experimental autoimmune encephalomyelitis. Am. J. Pathol. 160:271-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metz, L. M., S. D. McGuinness, and C. Harris. 1998. Urinary tract infections may trigger relapse in multiple sclerosis. Axone 19:67-70. [PubMed] [Google Scholar]
- 24.Morimoto, K., D. C. Hooper, A. Bornhorst, S. Corisdeo, M. Bette, Z. F. Fu, M. K. Schafer, H. Koprowski, E. Weihe, and B. Dietzschold. 1996. Intrinsic responses to Borna disease virus infection of the central nervous system. Proc. Natl. Acad. Sci. USA 93:13345-13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi, K. 2001. Innate and acquired activation pathways in T cells. Nat. Immunol. 2:140-142. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 27.Noda, S., K. Tanaka, S. Sawamura, M. Sasaki, T. Matsumoto, K. Mikami, Y. Aiba, H. Hasegawa, N. Kawabe, and Y. Koga. 2001. Role of nitric oxide synthase type 2 in acute infection with murine cytomegalovirus. J. Immunol. 166:3533-3541. [DOI] [PubMed] [Google Scholar]
- 28.Noske, K., T. Bilzer, O. Planz, and L. Stitz. 1998. Virus-specific CD4+ T cells eliminate Borna disease virus from the brain via induction of cytotoxic CD8+ T cells. J. Virol. 72:4387-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orange, J., and C. A. Biron. 1996. Characterization of early IL-12, IFN-alpha-beta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 156:4746-4756. [PubMed] [Google Scholar]
- 30.Orange, J. S., and C. A. Biron. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 156:1138-1142. [PubMed] [Google Scholar]
- 31.Pagenstecher, A., S. Lassmann, M. J. Carson, C. L. Kincaid, A. K. Stalder, and I. L. Campbell. 2000. Astrocyte-targeted expression of IL-12 induces active cellular immune responses in the CNS and modulates experimental allergic encephalomyelitis. J. Immunol. 164:4481-4492. [DOI] [PubMed] [Google Scholar]
- 32.Panitch, H. S. 1994. Influence of infection on exacerbations of multiple sclerosis. Ann. Neurol. 36(Suppl.):S25-S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, J. H., and S. H. Shin. 1996. Induction of IL-12 gene expression in the brain in septic shock. Biochem. Biophys. Res. Commun. 224:391-396. [DOI] [PubMed] [Google Scholar]
- 34.Planz, O., and L. Stitz. 1999. Borna disease virus nucleoprotein (p40) is a major target for CD8+-T-cell-mediated immune response. J. Virol. 73:1715-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richt, J., L. Stitz, U. Deschl, K. Frese, and R. Rott. 1990. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J. Gen. Virol. 71:2565-2573. [DOI] [PubMed] [Google Scholar]
- 36.Richt, J. A., L. Stitz, H. Wekerle, and R. Rott. 1989. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J. Exp. Med. 170:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 38.Sauder, C., W. Hallensleben, A. Pagenstecher, S. Schneckenburger, L. Biro, D. Pertlik, J. Hausmann, M. Suter, and P. Staeheli. 2000. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J. Virol. 74:9267-9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal, B. M., B. K. Dwyer, and E. M. Shevach. 1998. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 187:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sibley, W. A., C. R. Bamford, and K. Clark. 1985. Clinical viral infections and multiple sclerosis. Lancet i:1313-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 42.Stalder, A., A. Pagenstecher, C. Kincaid, and I. L. Campbell. 1999. Analysis of gene expression by multi-probe RNase protection assay, p. 53-66. In J. Harry and H. A. Tilson (ed.), Neurodegeneration methods and protocols, vol. 22. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 43.Stalder, A. K., A. Pagenstecher, N. C. Yu, C. Kincaid, C.-S. Chiang, M. V. Hobbs, F. E. Bloom, and I. L. Campbell. 1997. Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J. Immunol. 159:1344-1351. [PubMed] [Google Scholar]
- 44.Stitz, L., M. Sobbe, and T. Bilzer. 1992. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J. Virol. 66:3316-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trinchieri, G. 1998. Immunobiology of interleukin-12. Immunol. Res. 17:269-278. [DOI] [PubMed] [Google Scholar]
- 46.Vollstedt, S., M. Franchini, G. Alber, M. Ackermann, and M. Suter. 2001. Interleukin-12- and gamma interferon-dependent innate immunity are essential and sufficient for long-term survival of passively immunized mice infected with herpes simplex virus type 1. J. Virol. 75:9596-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldburger, K. E., R. C. Hastings, R. G. Schaub, S. J. Goldman, and J. P. Leonard. 1996. Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms. Am. J. Pathol. 148:375-382. [PMC free article] [PubMed] [Google Scholar]
- 48.Windhagen, A., J. Newcombe, F. Dangond, C. Strand, M. N. Woodroofe, M. L. Cuzner, and D. A. Hafler. 1995. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J. Exp. Med. 182:1985-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, J., H. Zhu, T. L. Murphy, W. Ouyang, and K. M. Murphy. 2001. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat. Immunol. 2:157-164. [DOI] [PubMed] [Google Scholar]