Abstract
TREK-1 (KCNK2) is a K2P channel that is highly expressed in fetal neurons. This K+ channel is opened by a variety of stimuli, including membrane stretch and cellular lipids. Here, we show that the expression of TREK-1 markedly alters the cytoskeletal network and induces the formation of actin- and ezrin-rich membrane protrusions. The genetic inactivation of TREK-1 significantly alters the growth cone morphology of cultured embryonic striatal neurons. Cytoskeleton remodelling is crucially dependent on the protein kinase A phosphorylation site S333 and the interactive proton sensor E306, but is independent of channel permeation. Conversely, the actin cytoskeleton tonically represses TREK-1 mechano-sensitivity. Thus, the dialogue between TREK-1 and the actin cytoskeleton might influence both synaptogenesis and neuronal electrogenesis.
Keywords: actin cytoskeleton, K2P channels, mechano-gating, PKA phosphorylation, KCNK
Introduction
The K2P channel TREK-1 is predominantly expressed in the central and peripheral nervous system where it is thought to have a dominant role in cell electrogenesis (Fink et al, 1996; Heurteaux et al, 2004). A particularly important functional property of TREK-1 is its mechanosensitivity, with channel opening induced by membrane stretch and cell swelling (Patel et al, 1998; Maingret et al, 1999; Honoré et al, 2002). TREK-1 is also reversibly activated by intracellular acidosis that sensitizes channel mechano-gating (Maingret et al, 1999; Honoré et al, 2002). Additionally, various cellular lipids reversibly stimulate TREK-1 opening (Patel et al, 1998; Maingret et al, 2000b; Chemin et al, 2005a, 2005b).
Earlier work has shown the functional expression of a mechano-gated and lipid-sensitive K+ channel that shares all the properties of TREK-1 in the growth cones of developing neurons, suggesting that this channel may have a functional role during axonal guidance and pathfinding (Belardetti et al, 1986; Sigurdson & Morris, 1989; Vandorpe & Morris, 1992).
Mutagenesis studies have shown that the cytosolic carboxy-terminal domain of TREK-1 is critical for TREK-1 stimulation by both physical and chemical stimuli (Patel et al, 1998, 1999; Maingret et al, 1999, 2000a, 2000b; Honoré et al, 2002; Chemin et al, 2005b). E306 and S333 are key residues in this region that control TREK-1 gating (Patel et al, 1998; Honoré et al, 2002; Chemin et al, 2005b). Protonation of E306 increases the interaction of the cytosolic C-terminal domain with the plasma membrane and leads to channel activation in the absence of membrane stretch (Honoré et al, 2002; Chemin et al, 2005). By contrast, phosphorylation of S333 is responsible for the protein kinase A (PKA)-mediated inhibition of TREK-1 (Patel et al, 1998). The E306A mutation prevents channel downmodulation by PKA phosphorylation of S333, which suggests that both sites are functionally linked (Honoré et al, 2002).
In the present report, we show that the expression of TREK-1 has profound effects on the actin network architecture in transfected cells and neurons. This effect is dependent on both the PKA phosphorylation site S333 and the functionally interactive proton sensor E306. Conversely, the actin cytoskeleton represses TREK-1 mechano-gating. The cross-talk between TREK-1 and the cytoskeleton may thus have an important role in the control of both synaptogenesis and neuronal excitability.
Results
TREK-1 alters the morphology of neurons
EYFP (enhanced yellow fluorescent protein) and EYFP–TREK-1 were expressed in rat neonatal hippocampal neurons, using the Semliki forest virus (Lauritzen et al, 2003; Fig 1A and supplementary Fig 1A,B online). The tagged TREK-1 channel retains all the properties of the nontagged channel (see below). EYFP–TREK-1 expression induces a profound change in the morphology of these neurons as compared with EYFP alone (Fig 1A,B and supplementary Fig 1A,B online). Numerous filopodia-like structures are induced within both the dendritic tree and the axon (Fig 1A (lower panel) and supplementary Fig 1A,B online). Staining with the actin marker phalloidin shows a colocalization of TREK-1 and actin in these protrusions (supplementary Fig 1A,B online). The relative surface area of neurons expressing TREK-1 is almost double that of those expressing EYFP (Fig 1B). Next, we compared the morphology of the growth cones of cultured striatal embryonic (E14) neurons from wild type (WT) mice, which naturally express high levels of TREK-1, with that of knockout (KO) mice (Heurteaux et al, 2004; Fig 1C,D and supplementary Fig 2 online). The proportion of expanded growth cones is significantly reduced when TREK-1 is inactivated (Fig 1C,D and supplementary Fig 2 online).
Figure 1.
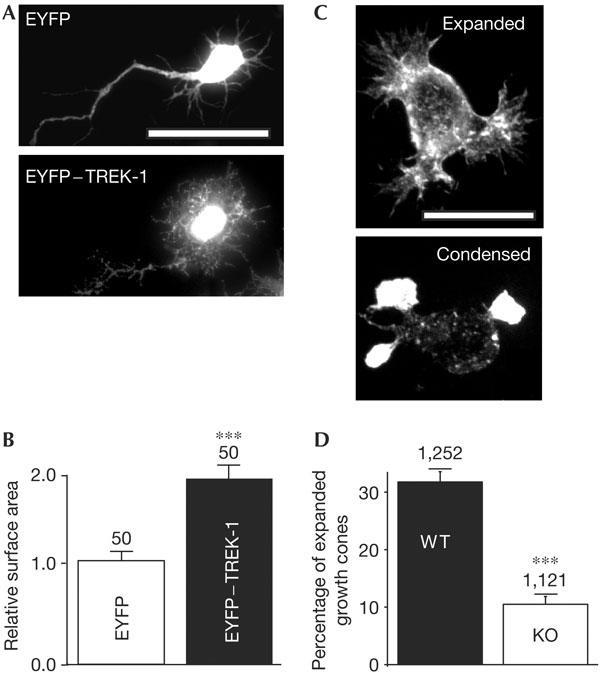
Expression of TREK-1 conditions the morphology of cultured neurons. (A) Young stage 2 hippocampal neurons were infected with Semliki forest virus bearing either EYFP (enhanced yellow fluorescent protein; top panel) or EYFP–TREK-1 (bottom panel). Scale bar, 20 μm. (B) A total of 50 individual neurons infected with each virus were randomly selected in a blind way and measured for surface area using the software ImageJ. The difference is significant (P<0.001). (C) Morphology of cultured embryonic striatal neurons from wild-type (WT) mice (top panel showing expanded growth cones) and TREK-1 knockout (KO) mice (bottom panel showing condensed growth cones). Neurons were stained with phalloidin. Scale bar, 10 μm. (D) Percentage of expanded growth cones in cultured neurons from TREK-1 WT (black column) and KO (white column) mice. Numbers of growth cones examined from four independent neuronal cultures (supplementary Fig 2 online) are indicated. The difference is significant (P<0.001).
TREK-1 induces the formation of filopodia-like structures
The effect of TREK-1 on the actin cytoskeleton is similarly observed in non-neuronal cells (Fig 2). COS cells were transiently transfected with either EYFP or EYFP–TREK-1. EYFP shows a diffuse cytosolic labelling, whereas EYFP–TREK-1 is localized to membrane protrusions present around the cell periphery and on the dorsal surface (Fig 2A,B). Costaining of EYFP–TREK-1-positive cells with phalloidin shows a perfect overlay in these membrane extensions (Fig 2B, right panels). Phalloidin labelling of EYFP-transfected cells shows the absence of such structures in control cells (Fig 2A, right panels). Thus, EYFP–TREK-1 not only localizes to, but also induces the formation of, filopodia-like structures (Fig 2A,B). Similar findings are obtained when cells are transfected with the non-tagged channel and detected with a polyclonal antibody directed against TREK-1 (supplementary Fig 1C online). There is no apparent alteration in the microtubule network (not shown). The protrusions seen in TREK-1-expressing cells are reminiscent of structures obtained with activated ERM (ezrin–radixin–moesin) proteins that link F-actin to specific membrane proteins (Bretscher et al, 2002). The structures observed in TREK-1-expressing cells are ezrin positive (Fig 2C). TREK-1 has a similar effect in transiently transfected NIH 3T3, HEK and CHO cells (not shown).
Figure 2.
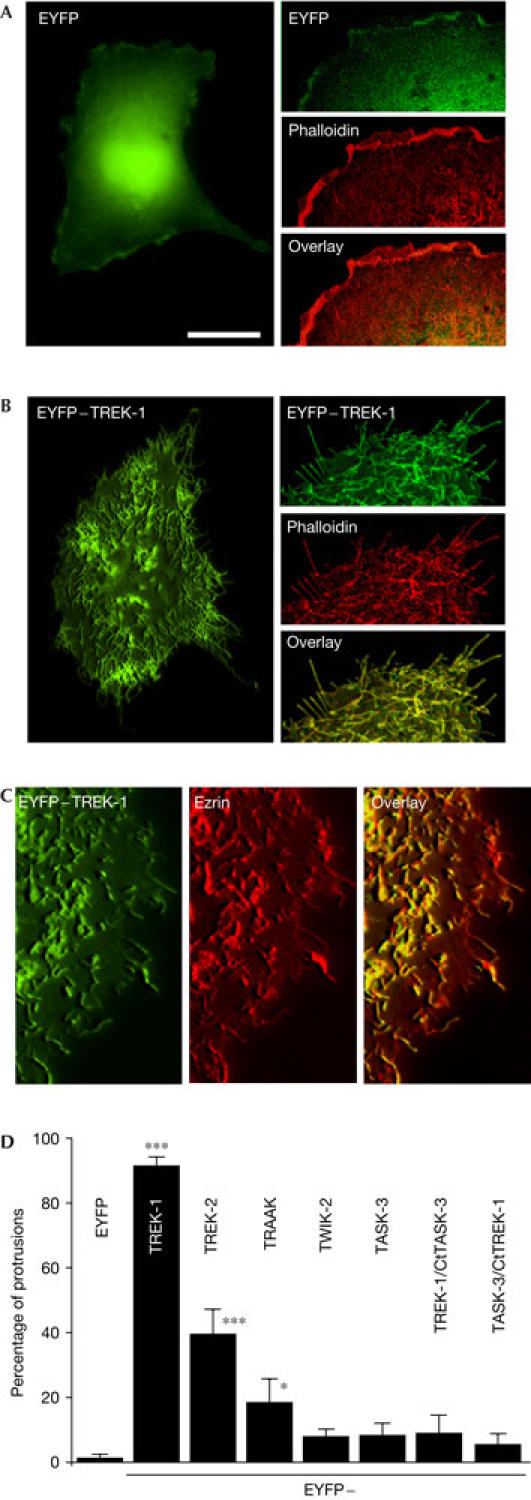
Induction of filopodia-like structures by expression of TREK-1 in COS7 cells. (A) Left panel: epifluorescence three-dimensional (3D) image of an EYFP (enhanced yellow fluorescent protein)-transfected cell. (B) Left panel: epifluorescence 3D image of an EYFP–TREK-1 transfected cell. (A,B) Right panels: confocal images of EYFP- or EYFP–TREK-1-transfected cells stained with Alexa Fluor® 594 phalloidin. The overlay between EYFP and phalloidin staining is shown in the bottom panel. (C) Epifluorescence images of an EYFP–TREK-1-expressing cell immunolabelled with an antibody against ezrin. Ezrin labelling was detected using goat anti-rabbit Alexa Fluor® 594. The overlay of EYFP–TREK-1 and ezrin is depicted on the right. (D) Quantification of COS7 cells transfected with various EYFP-tagged K2P channels and TREK-1/TASK-3 and TASK-3/TREK-1 chimaeras (see the supplementary information online). The histogram represents the percentage of cells in which channel proteins are localized to membrane protrusions. A total of 120 cells from three to four independent experiments were counted blind. Scale bars, 25 μm (A,B (left panels)) and 10 μm (A,B (right panels), C).
COS cells were also co-transfected with other EYFP-tagged K2P domain channels (Fig 2D). Biophysical and pharmacological properties of the channel chimaeras were unaltered by the presence of EYFP (data not shown). TREK-2 and TRAAK are seen in surface protrusions, although the density of such structures is largely reduced as compared with TREK-1. TWIK-2 and TASK-3 fail to alter cell morphology (Fig 2D). The chimaera TREK-1/CtTASK-3 does not induce microvilli and filopodia structures, suggesting that the cytosolic C-terminal domain not only has an important role in the regulation of channel gating, but is also involved in the morphogenic effect of TREK-1 (Fig 2D). However, fusing of the C-terminal domain of TREK-1 to the core of TASK-3 (linked to EYFP at the amino terminus) fails to induce microvilli formation (Fig 2D).
Cytoskeleton remodelling requires key residues in TREK-1
The C-terminal domain encompasses residues E306 and S333, which are key for TREK-1 gating. When E306 is converted to an alanine (E306A), the channel no longer requires stretch for activation (Honoré et al, 2002). Conversion of the PKA consensus site S333 to an alanine (S333A) renders TREK-1 resistant to cyclic AMP stimulation (Patel et al, 1998). The S333A mutant mimics the dephosphorylated form of TREK-1, whereas S333D mutant mimics the phosphorylated state of the channel (Patel et al, 1998; Bockenhauer et al, 2001). Cells expressing E306A or S333A show a smooth surface, with few peripheral or apical protrusions apparent (Fig 3A,C). Conversely, cells expressing E306D or S333D are indistinguishable from those expressing the WT channel (Fig 3B,D). Both the E306D and S333D channels, unlike E306A and S333A, show a clear colocalization with actin within filopodia (Fig 3B,D). Wheat germ agglutinin (WGA) labelling of the cell membrane confirms that formation of membrane protrusions is stimulated in WT TREK-1-, E306D- and S333D-expressing cells (Fig 4A–D). The fact that EYFP–TREK-1 and S333D give a similar morphological phenotype suggests that TREK-1 is endogenously phosphorylated under basal conditions. Incubation of cells with 300 μM CPT-cAMP does not change the number of cells with surface protrusions when expressing WT TREK-1 (data not shown).
Figure 3.
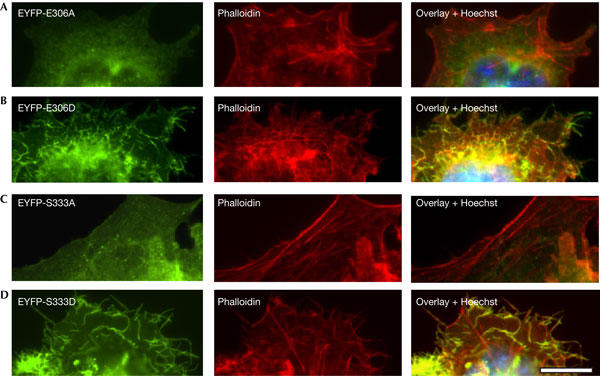
E306 and S333 mutations affect cytoskeleton remodelling. Epifluorescence images of COS7 cells transfected with (A) EYFP (enhanced yellow fluorescent protein)-E306A, (B) EYFP-E306D, (C) EYFPs333A and (D) EYFP-S333D. Cells were co-stained with Alexa Fluor® 594 phalloidin. Overlay images show colocalization of EYFP-E306D and EYFP-S333D mutants with actin in cell protrusions. Scale bar, 15 μm.
Figure 4.
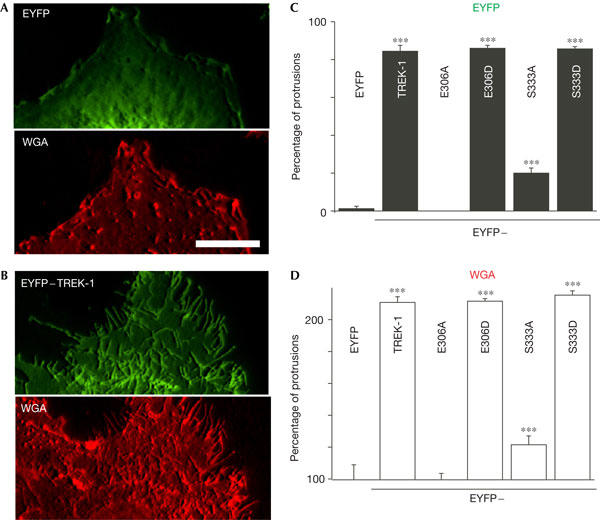
The proton sensor E306 and the protein kinase A site S333 are crucial for the morphogenic effect of TREK-1. (A) EYFP (enhanced yellow fluorescent protein) and Alexa Fluor® 594 wheat germ agglutinin (WGA) fluorescence of an EYFP-transfected cell. (B) The same experiment with an EYFP–TREK-1-expressing cell. (C) COS7 cells were transfected with EYFP, EYFP–TREK-1 or different point mutants of TREK-1 fused to EYFP. The percentage of cells with channels in protrusions was quantified by examining the EYFP fluorescence. In (C,D), 160 cells from four independent experiments were counted blind. (D) The same cells were examined and the percentage of cells with protrusions was determined using WGA fluorescence. The values are normalized to the control EYFP condition. Scale bar, 10 μm.
Because the mutants E306A and S333A show a much higher channel activity as compared with WT TREK-1 and S333D (see below), it was important to determine whether channel activity is required for the morphogenic effect. Cells expressing TREK-1 were grown in the presence of either 75 mM K+, to reduce K+ efflux, or the potent TREK-1 inhibitor cationic lipid oleylamin (5 μM), to fully inhibit TREK-1 channel activity (supplementary Fig 3 online). In both cases, the formation of actin protrusions by TREK-1 was not altered, indicating that channel activity is not necessary for the morphogenic effect (supplementary Fig 3 online).
E306A, S333A and cytoskeleton interaction
In the cell-attached patch configuration at atmospheric pressure, basal channel activity is absent or very low for EYFP–TREK-1 and S333D (Fig 5A). Both S333A and E306A show a much higher activity in the absence of pressure stimulation (Fig 5A, 0 mmHg). Membrane stretch gradually increases TREK-1 channel activity, but has little effect on E306A that is constitutively open (Fig 5A). The pressure–effect curve of S333A (P0.5: −39 mmHg) is shifted towards more positive pressure as compared with WT TREK-1 (P0.5: −62 mmHg) and S333D (P0.5: −61 mmHg; Fig 5A). Activation of WT TREK-1 and S333D by stretch is potentiated by pretreatment with latrunculin A, which disrupts actin polymerization, whereas the microtubule-disrupting agent nocodazole has little effect (Fig 5B,D). At −60 mmHg, S333A behaves like E306A and is essentially insensitive to latrunculin A (Fig 5B). Patch excision in the inside-out configuration strongly increases TREK-1 channel sensitivity to membrane stretch (Fig 5C–E). This effect is observed with WT TREK-1 and S333D, but is absent with S333A and E306A (Fig 5C–E). After treatment with latrunculin A in the cell-attached configuration, potentiation by patch excision of WT TREK-1 and S333D is also absent, which suggests that patch excision similarly disrupts the actin cytoskeleton (Fig 5E). Taken together, these results suggest that both S333 and E306 regulate the interaction of TREK-1 with the cytoskeleton and control channel mechano-gating.
Figure 5.
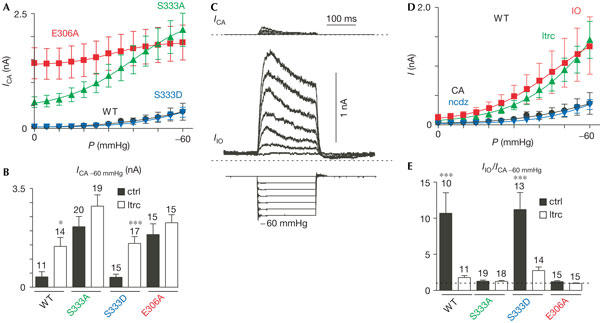
The actin cytoskeleton tonically represses TREK-1 channel mechano-gating. (A) Channel activity of WT TREK-1 (wild type) was monitored in the cell-attached patch configuration at a holding potential of 0 mV. The activity of WT TREK-1 (black, n=12), S333D (blue, n=15), S333A (green, n=20) and E306A (red, n=15) was compared at increasing negative pressure values. Pressure–effect curves were fitted with Boltzmann relationships with P0.5 of −62.4, −60.9, −39.5 and −31.5 and slope factors k of 15.9, 11.7, 14.0 and 8.8 for WT, S333D, S333A and E306A, respectively. (B) The activity of TREK-1 elicited by a membrane stretch of −60 mmHg at 0 mV was monitored in the cell-attached configuration in the absence (ctrl in the presence of dimethyl sulphoxide (DMSO)) and in the presence of 3 μM latrunculin A (ltrc) for 1 h (white bars). (C) TREK-1 channel activity was monitored in the cell-attached configuration (top traces) and 2 min after excision in the inside-out patch configuration (middle traces) at increasing negative pressures from 0 to −60 mmHg (bottom traces) at a holding potential of 0 mV. (D) TREK-1 channel activity was monitored in the cell-attached patch configuration in the control condition containing DMSO (black, n=12), after treatment for 1 h with 3 μM latrunculin A (green, n=15), after 4 h of treatment with 10 μM nocodazole (blue, n=10) and after patch excision in the inside-out patch configuration (red, n=10) at 0 mV. Pressure–effect curves were fitted with Boltzmann relationships with P0.5 values of −62.4, −70.7, −65.1 and −46.1 mmHg and a slope factor k of 15.9, 16.6, 12.2 and 13.9 for WT TREK-1 cell-attached patch configuration, with latrunculin A, with nocodazole and after excision in the inside-out configuration, respectively. (E) Effect of membrane excision in the inside-out patch configuration before and after 1 h of treatment with 3 μM latrunculin A (white bars) on WT TREK-1, S333D, S333A and E306A mutants at 0 mV.
Discussion
Our data provide evidence that TREK-1 shapes the actin cytoskeleton. Importantly, TREK-1-influenced cytoskeletal remodelling does not depend on channel activity, as it is not affected by the presence of a concentration of oleylamin that fully inhibits TREK-1 or by high external K+ that reduces K+ efflux. Expression of the α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptor subunit GluR2 has been shown to similarly induce dendritic spines in neurons independently of its channel properties (Passafaro et al, 2003). The mutations E306A and S333A that strongly increase channel activity suppress the effect of TREK-1 on the cytoskeleton. Thus, there is an inverse relationship between channel activity and the morphogenic effect of TREK-1.
Although residues S333 and E306 have a crucial role in the induction of actin protrusions, the exact mechanism by which this occurs remains obscure. ERM proteins, and ezrin in particular, are known to be involved in the formation of microvilli in a large number of cell types (Bretscher et al, 2002). As TREK-1 is extensively colocalized with ezrin in filopodia-like structures, it is possible that TREK-1 might interact with ERM proteins and/or the adaptor protein EBP50 and could be involved in the activation and translocation of this actin adapter to the membrane. However, it still remains unknown whether such an interaction occurs.
In a previous report, we expressed the isolated C-terminal domain of TREK-1 fused to EYFP in transfected COS cells (Chemin et al, 2005b). This construct was localized at the plasma membrane, although no membrane protrusions were observed (Chemin et al, 2005b). Similarly, expression of a chimaera between the core of TASK-3 and the C-terminal domain of TREK-1 fails to affect cell morphology (Chemin et al, 2005b). These negative results show that the C-terminal domain of TREK-1 is necessary, but not sufficient, to induce a morphologic effect.
For many cellular processes, it is essential that reversible protein phosphorylation be tightly regulated. Protein phosphatases and kinases are associated with regulatory subunits that not only target these enzymes to specific subcellular compartments, but also determine substrate specificity (Ceulemans & Bollen, 2004). The phospho-mimetic mutant S333D induces actin protrusions, whereas the dephosphorylated mutant S333A has only a weak effect. The presence of a regulated receptor-coupled kinase and/or phosphatase, targeted to the same regions as TREK-1, could serve to dynamically regulate the electrophysiological and morphological activities of TREK-1 on demand.
TREK-1 was proposed to be the functional mammalian homologue of the Aplysia S-type K+ channel that is found in the growth cones of sensory neurons (Belardetti et al, 1986; Sigurdson & Morris, 1989; Vandorpe & Morris, 1992; Patel et al, 1998). The presence of TREK-1 in the growth cones could serve to hyperpolarize the membrane and modulate calcium entry, which is important for both axonal guidance and pathfinding. Moreover, a morphological role for TREK-1, independently of its channel function, as shown in the present report, may also be at play. The strong expression of TREK-1 in the human fetal brain as compared with that in the adult (Medhurst et al, 2001) and the alteration in growth cone morphology of neurons from TREK-1 KO mice (the present study) suggest a possible role for this channel during early neuronal development. Thus, TREK-1 could have a significant role in axonal migration and synaptogenesis.
Membrane excision markedly sensitizes TREK-1 (the present study) and the native S channel to membrane stretch (Small & Morris, 1994; Wan et al, 1999). Furthermore, the stretch-induced activity depends on the mechanical history of the patch (E.H. & Sachs, unpublished; Small & Morris, 1994; Wan et al, 1999). These effects are attributed to a tonic repression of TREK-1 by the actin cytoskeleton, which is physically disrupted by patch excision. One possible mechanism may involve a direct interaction with the channel protein. Another alternative is that the actin network prevents bilayer deformation during pressure stimulation. Further work will be required to investigate these different possibilities. Thus, the regulation of TREK-1 mechano-gating by the actin cytoskeleton will strongly influence the electrophysiological role of this K+ channel.
In conclusion, we have shown cross-talk between the K+ channel TREK-1 and the actin cytoskeleton. We show the key role of the proton sensor E306 and the PKA phosphorylation site S333 in the reciprocal regulation between the channel and the actin cytoskeleton. It will be important to investigate the respective contribution of the electrophysiological and morphological effects of TREK-1 to the phenotype of the KO mice, including vulnerability to brain ischaemia and epilepsy as well as resistance to volatile general anaesthetics (Heurteaux et al, 2004).
Methods
Electrophysiological recordings. An N-terminal EYFP-tagged mTREK-1 channel was used and no functional difference with non-tagged WT channel was observed, including sensitivity to membrane stretch, excision and cytoskeletal disrupting agents (Patel & Honoré, 2001). Amino-acid numbering is related to the mTREK-1 splice variant (GenBank U73488). For the electrophysiology of the COS cells, the external medium (pipette) contained 150 mM NaCl, 5 mM KCl, 3 mM MgCl2, 1 mM CaCl2 and 10 mM HEPES, pH 7.4 with NaOH. The cytosolic medium (bath) contained 155 mM KCl, 5 mM EGTA, 3 mM MgCl2 and 10 mM HEPES at pH 7.2 with KOH. Patch pipettes of about 1.5 MΩ were used for cell-attached and excised inside-out patches. Negative pressure was applied at the back of the patch pipette using a fast pressure clamp system (ALA fast pressure clamp system). Latrunculin A and nocodazole obtained from Sigma-Aldrich (Saint Quentin Fallavier, France) were dissolved at a concentration of 3 and 10 mM in dimethyl sulphoxide (DMSO), respectively. Control experiments were carried out with DMSO alone.
Molecular and cell biology methods. See supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank M. Arpin and P. Chardin for fruitful discussion. This work was supported by the CNRS, l'ARC, la Ligue contre le cancer and the Institute Paul Hamel.
References
- Belardetti F, Schacher S, Siegelbaum SA (1986) Action potentials, macroscopic and single channel currents recorded from growth cones of Aplysia neurones in culture. J Physiol 374: 289–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenhauer D, Zilberberg N, Goldstein SA (2001) KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci 4: 486–491 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599 [DOI] [PubMed] [Google Scholar]
- Ceulemans H, Bollen M (2004) Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84: 1–39 [DOI] [PubMed] [Google Scholar]
- Chemin J, Patel A, Duprat F, Zanzouri M, Lazdunski M, Honoré E (2005a) Lysophosphatidic acid-operated K+ channels. J Biol Chem 280: 4415–4421 [DOI] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E (2005b) A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J 24: 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J 15: 6854–6862 [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C et al. (2004) TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J 23: 2684–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E, Maingret F, Lazdunski M, Patel AJ (2002) An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J 21: 2968–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honoré E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ (2003) K+-dependent cerebellar granule neuron apoptosis: role of TASK leak K+ channels. J Biol Chem 278: 32068–32076 [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E (1999) Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem 274: 26691–26696 [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel A, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honoré E (2000a) TREK-1 is a heat-activated background K+ channel. EMBO J 19: 2483–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E (2000b) Lysophospholipids open the two P domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem 275: 10128–10133 [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, Gloger II, Pangalos MN (2001) Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Mol Brain Res 86: 101–114 [DOI] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M (2003) Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature 424: 677–681 [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E (2001) Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci 24: 339–346 [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17: 4283–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M (1999) Inhalational anaesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2: 422–426 [DOI] [PubMed] [Google Scholar]
- Sigurdson WJ, Morris CE (1989) Stretch-activated ion channels in growth cones of snail neurons. J Neurosci 9: 2801–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DL, Morris CE (1994) Delayed activation of single mechanosensitive channels in Lymnaea neurons. Am J Physiol 267: C598–C606 [DOI] [PubMed] [Google Scholar]
- Vandorpe DH, Morris CE (1992) Stretch activation of the Aplysia S-channel. J Membr Biol 127: 205–214 [DOI] [PubMed] [Google Scholar]
- Wan X, Juranka P, Morris CE (1999) Activation of mechanosensitive currents in traumatized membrane. Am J Physiol 276: C318–C327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


