Abstract
This study examined whether small ubiquitin-related modifier-1 (SUMO-1) regulates apoptosis signal-regulating kinase 1 (ASK1). ASK1 interacted with SUMO-1 in vitro as well as in BOSC23 cells. Endogenous ASK1–SUMO-1 interaction was disrupted following H2O2 signal. SUMO-1 overexpression suppressed the self-oligomerization, kinase activity and apoptotic potential of ASK1, whereas SUMO-1 depletion potentiated such activities. SUMO-1(ΔC6), a sumoylation-incompetent mutant lacking carboxy-terminal six amino acids, suppressed ASK1 activation, implying that the suppressive effect of SUMO-1 on ASK1 is independent of sumoylation. ASK1(3M), an ASK1 mutant in which all three lysines in the ψKXE motif were substituted with alanines, still retained the kinase activity and activated the Jun amino-terminal kinase pathway. However, SUMO-1 failed to interact with ASK1(3M) and to suppress ASK1(3M) activation, indicating that the three lysines are important for regulation by SUMO-1. This study shows that SUMO-1 exerts a negative regulatory effect on ASK1 activation through physical interaction and not through covalent modification.
Keywords: ASK1, interaction, SUMO-1
Introduction
Apoptosis signal-regulating kinase (ASK1) is an upstream activator of Jun amino-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) signalling cascades (Davis, 2000). Activation of ASK1 triggers diverse biological responses and has several binding partners. ASK1 binds to death-receptor-associated proteins, such as TRAFs, Daxx and hD53L1, and leads to MAPK activation (Cho et al, 2004). ASK1 activation can be inhibited by interactions with 14-3-3, thioredoxin, HSP-72, Nef and Raf-1 (Chen et al, 2001; Goldman et al, 2004).
Covalent modification by small ubiquitin-related modifier (SUMO)—sumoylation—regulates diverse cellular processes (Muller et al, 2001). Sumoylation is often targeted to lysines in the ψKXE motif (where ψ represents hydrophobic amino acids and X is any amino acid; Sampson et al, 2001). However, lysines that are not embedded in the ψKXE motif can also be targeted by SUMO (Johnson & Blobel, 1999; Kim et al, 1999). Sumoylation accumulates Ran GTPase-activating protein (RanGAP1) at the nuclear pore (Matunis et al, 1998) and transports Sp100 to promyelocytic leukaemia (PML) nuclear bodies (Zhong et al, 2000). Modification of transcription factors, including those in the p53, c-Jun, Sp3, c-Myb and C/EBP families, by SUMO-1 reduces its transcriptional activity (Gill, 2004). Sumoylation stabilizes CREB, MDM2 and IκBα by blocking ubiquitination (Muller et al, 2001). SUMO-1 also modulates cytokines, Wnt, AP-1 and steroid hormone signalling pathways by conjugating with STAT1, LEF-1, c-Jun, Elk-1 and androgen receptor (Muller et al, 2004).
SUMO-1 can regulate cellular processes without the characteristic conjugation. SUMO-1 inhibits RAD51-mediated homologous recombination by interaction with RAD51 (Li et al, 2000). SUMO-1 interacts with Fas and tumour necrosis factor receptor 1 (TNFR1) and provides protection against cell death, although covalent modifications of Fas and TNFR1 have not been observed (Okura et al, 1996). SUMO-1 can also inhibit dynamin-dependent endocytosis without the covalent modification of dynamin (Mishra et al, 2004).
In the present study, we explored the involvement of SUMO-1 in ASK1-mediated signalling. SUMO-1 interacts with ASK1 and inhibits ASK1 activation. Results show that physical interaction is sufficient for SUMO-1 to suppress ASK1 activation.
Results
ASK1 interacts with SUMO-1 and Ubc9
We examined whether ASK1 interacted with SUMO-1 in vitro. As Ubc9 binds to the SUMO-1 acceptor site and efficiently transfers SUMO-1 to targets, we also examined whether ASK1 interacted with Ubc9. 35S-labelled ASK1 was produced by in vitro translation and was incubated with purified glutathione S-transferase (GST), GST–Ubc9 or GST–SUMO-1 immobilized on GST–agarose beads (supplementary Fig S1 online). SUMO-1 and Ubc9 proteins interacted with ASK1 in vitro. Interactions of ASK1 with SUMO-1 and Ubc9 were also examined in BOSC23 cells. Xpress–SUMO-1 protein was detected in the immunoprecipitated haemagglutinin (HA)–ASK1 complexes, showing that ASK1–SUMO-1 interaction could occur in BOSC23 cells (Fig 1A, left panel). Anti-ASK1 antibody immunoprecipitated both ASK1 and SUMO-1 from BOSC23 cell lysates, indicating endogenous interaction (Fig 1A, right panel). However, the endogenous ASK1–SUMO-1 interaction was disrupted markedly by H2O2 treatment, implying that the interaction could be regulated physiologically. A similar set of experiments was carried out for ASK1–Ubc9 interaction. Flag–Ubc9 protein was also detected in the immunoprecipitated HA–ASK1 complexes (Fig 1B, left panel). Endogenous interaction between ASK1 and Ubc9 was also observed (Fig 1B, right panel) and the interaction was sustained by H2O2 treatment. Therefore, the interaction of ASK1 with SUMO-1 is regulated by oxidative stress, whereas that of Ubc9 with BOSC23 cells is not.
Figure 1.

Apoptosis signal-regulating kinase 1 interacts with Ubc9 and small ubiquitin-related modifier-1 in BOSC23 cells. (A) Apoptosis signal-regulating kinase 1 (ASK1) binds to small ubiquitin-related modifier-1 (SUMO-1) in BOSC23 cells. Cells were co-transfected with 2 μg of indicated DNAs. At 48 h after transfection, cell lysates were immunoprecipitated with anti-haemagglutinin (HA) antibody and immunoblotted with anti-Xpress antibody (left panel). BOSC23 cells were untreated or treated for 1 h with 1 mM H2O2. Cell lysates were immunoprecipitated with anti-mouse IgG1 or with anti-ASK1 antibody and were immunoblotted with antisUMO-1 antibody (right panel). Whole-cell lysates (WCLs) were also immunoblotted with the indicated antibodies to show the expression. (B) ASK1 binds to Ubc9 in BOSC23 cells. A similar set of experiments as in (A) was carried out using Flag–Ubc9 instead of Xpress–SUMO-1 (left panel) and Ubc9 antibody instead of SUMO-1 antibody (right panel). IP, immunoprecipitation; WB, western blot.
ASK1 sumoylation was not detected in BOSC23 cells
The possibility that ASK1 might be covalently modified by SUMO-1 was examined because ASK1 contains three lysine residues (K535, K1084 and K1115) embedded in ψKXE, the sumoylation consensus motif (Fig 2A). BOSC23 cells were transiently transfected with HA–ASK1, Flag–Ubc9, MaxA–Xpress–SUMO-1 and MaxA–Xpress–SUMO-1(ΔC6), sumoylation-incompetent mutant lacking carboxy-terminal six amino acids, with the indicated combinations (Fig 2B). Sumoylated Flag–PML proteins (marked with asterisks) were clearly observed when SUMO-1 was co-transfected. SUMO-1(ΔC6) failed to show PML sumoylation as expected (Fig 2B, right panel). Both anti-ASK1 and anti-Xpress antibodies failed to detect putative sumoylated forms of ASK1 under our experimental conditions (Fig 2B, left panel).
Figure 2.
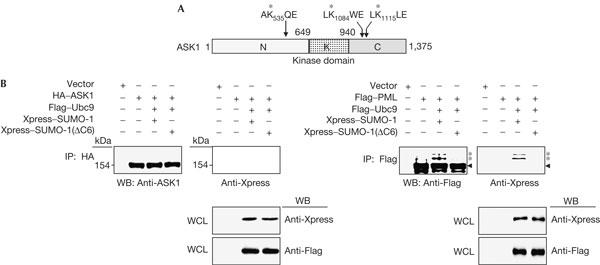
Covalent modification of apoptosis signal-regulating kinase 1 by small ubiquitin-related modifier-1 is not observed. (A) Schematic representation of apoptosis signal-regulating kinase 1 (ASK1) indicating the amino-terminal domain (N), the kinase domain (K), the carboxy-terminal domain (C) and the ψKXE motif (indicated by arrows). The K's marked with asterisks are the lysines in the ψKXE motifs, and are located at amino-acid numbers 535, 1084 and 1115, respectively. (B) BOSC23 cells were co-transfected with 2 μg of indicated plasmids. At 48 h after transfection, cell lysates were immunoprecipitated with anti-haemagglutinin (HA) antibody and immunoblotted with anti-ASK1 antibody and anti-Xpress antibody. Whole-cell lysates (WCLs) were immunoblotted with the indicated antibodies to show the expression. As a control, an experiment similar to that shown in the left panel was performed, except that promyelocytic leukaemia (PML) protein was used instead of ASK1 (right panel). IP, immunoprecipitation; WB, western blot.
ASK1(3M) retains kinase activity
To know whether lysines in the ψKXE motif affect the kinase activity of ASK1, ASK1(3M)—in which lysines at 535, 1084 and 1115 were substituted with alanines—was analysed for its kinase activity and self-oligomerization potential. Similar to wild-type ASK1, ASK1(3M) phosphorylated known substrates MAPK kinase 6 (MKK6) and SAPK/ERK kinase 1 (SEK1), as well as itself following H2O2 challenge, implying that kinase activity was not impaired (Fig 3A). The oligomerization potential of ASK1(3M) also remained intact (Fig 3B). Thus, lysines in the ψKXE motif did not affect the kinase activity of ASK1.
Figure 3.

Apoptosis signal-regulating kinase 1 lysines in the ψKXE motifs are not essential for the kinase activity and oligomerization potential. (A) BOSC23 cells were transfected for 48 h with 2 μg of either haemagglutinin (HA)–apoptosis signal-regulating kinase 1 (ASK1) or HA–ASK1(3M) (an ASK1 mutant in which all three lysines in the ψKXE motif were substituted with alanines). Cells were treated for 1 h with 1 mM H2O2 and cell lysates were immunoprecipitated with anti-HA antibody and assayed for the kinase activity in vitro. First panel, autophosphorylation assay of ASK1; second and third panels, in vitro kinase assay using glutathione S-transferase (GST)–mitogen-activated protein kinase kinase 6 (MKK6) and GST–SEK1 as substrates, respectively; fourth panel, whole-cell lysates (WCLs) were immunoprecipitated and immunoblotted with anti-HA antibody. (B) A 2 μg portion of Flag–ASK1, HA–ASK1 and HA–ASK1(3M) was transfected with the indicated combinations in BOSC23 cells. At 48 h after transfection, cell lysates were immunoprecipitated with anti-Flag antibody and the resulting precipitates were immunoblotted with anti-HA antibody (top panel). WCLs were immunoblotted with the indicated antibodies to show the expression. IP, immunoprecipitation; WB, western blot.
ASK1(3M) fails to interact with SUMO-1
As the three lysines did not affect ASK1 kinase activity, we next examined whether they influenced ASK1–SUMO-1 interaction. The three lysines in ASK1 were involved in the binding of ASK1 to SUMO-1 (supplementary Fig S2 online). GST pull-down assay showed that SUMO-1 and SUMO-1(ΔC6) could interact with ASK1 but barely interacted with ASK1(3M) (Fig 4A). However, Ubc9 could interact with both ASK1 and ASK1(3M). These results indicate that the three lysines of ASK1 are indispensable, whereas the C terminus of SUMO-1, which is essential for sumoylation, is dispensable for ASK1–SUMO-1 binding. The interaction was also investigated in the mammalian cellular environment (Fig 4B). Both Xpress–SUMO-1 and Xpress–SUMO-1(ΔC6) proteins were detected in the pull-down HA–ASK1 complexes, but not in the pull-down HA–ASK1(3M) complexes. These results show that lysine residues are essential in the in vivo interaction between ASK1 and SUMO-1.
Figure 4.
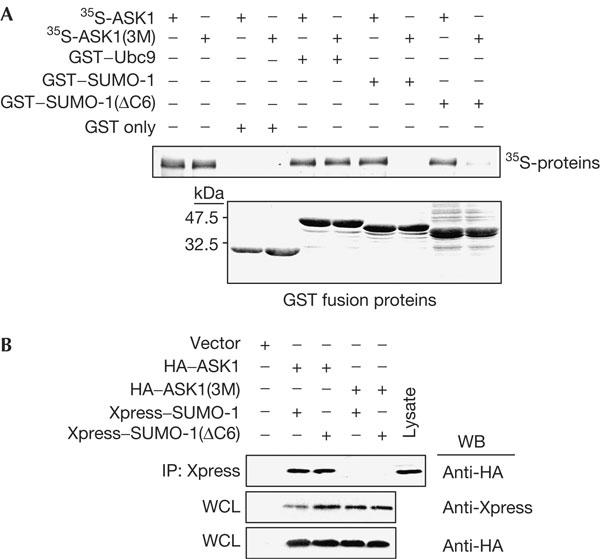
Lysines in the ψKXE motifs of apoptosis signal-regulating kinase 1 are important for the interaction with small ubiquitin-related modifier-1. (A) Glutathione S-transferase (GST) fusion proteins immobilized on glutathione–Sepharose 4B beads were incubated with 35S-labelled apoptosis signal-regulating kinase 1 (35S-ASK1) and 35S-labelled ASK1(3M) (35S-ASK1(3M), an ASK1 mutant in which all three lysines in the ψKXE motif were substituted with alanines). The pull-down complexes were separated on an SDS–polyacrylamide gel electrophoresis gel and analysed by autoradiography (upper panel). The lower panel shows Coomassie staining of the GST fusion proteins. (B) BOSC23 cells were transfected with 2 μg of indicated plasmids. At 48 h after transfection, cell lysates were immunoprecipitated with anti-Xpress antibody and the resulting precipitates were immunoblotted with anti-haemagglutinin (HA) antibody (top panel). Whole-cell lysates (WCLs) were immunoblotted with the indicated antibodies to show the expression. IP, immunoprecipitation; WB, western blot.
SUMO-1 inhibits ASK1 activation
We examined whether ASK1–SUMO-1 interaction affected ASK1 kinase activity. The efficiency of SUMO-1 depletion is shown using short interfering RNA (siRNA; Fig 5A). siRNA–SUMO-1 depleted SUMO-1 in a time-dependent manner, whereas β-tubulin remained unchanged. The control small cytoplasmic RNA (scRNA), a 21-nucleotide RNA oligonucleotide with no significant homology to any mammalian gene sequence (Zhang et al, 2004b), did not affect SUMO-1 expression. The anti-HA antibody pulled down both HA–ASK1 and Flag–ASK1, indicating the oligomerization of ASK1 (Fig 5B). However, this oligomerization was disrupted by transfection of either SUMO-1 or SUMO-1(ΔC6). This implies that inhibition of ASK1 oligomerization occurs independently of the covalent modification by SUMO-1. Such inhibition of ASK1 oligomerization by SUMO-1 and SUMO-1(ΔC6) was not observed in the pull-down ASK1(3M) complexes. The oligomerization potential of ASK1 was enhanced by SUMO-1 depletion.
Figure 5.

Small ubiquitin-related modifier-1 suppresses the oligomerization potential, kinase activity and Jun N-terminal kinase activation potential of apoptosis signal-regulating kinase 1. (A) A 2 μg portion of small cytoplasmic RNA (scRNA) or short interfering RNA (siRNA)–small ubiquitin-related modifier-1 (SUMO-1) was transfected into BOSC23 cells. Transfected cells were incubated for the indicated periods. Cell lysates were immunoblotted with antisUMO-1 antibody (top panel). β-Tubulin was used as a protein loading control (lower panel). (B) A 2 μg portion of plasmids with or without siRNA–SUMO-1 was transfected into BOSC23 cells with the indicated combinations. At 72 h after transfection, cell lysates were immunoprecipitated with anti-haemagglutinin (HA) antibody, and the resulting precipitates were immunoblotted with either anti-Flag antibody or anti-HA antibody (top two panels). Whole-cell lysates (WCLs) were also immunoblotted with the indicated antibodies to show the expression. (C) BOSC23 cells were transfected with 2 μg of plasmids with the indicated combinations. At 72 h after transfection, cell lysates were immunoprecipitated with anti-HA antibody and were assayed for autophosphorylation and kinase activity in vitro. Top panel, autophosphorylation of ASK1; second and third panels, in vitro kinase assays using glutathione S-transferase (GST)–mitogen-activated protein kinase kinase 6 (MKK6) and GST–SEK1 as substrates; WCLs were immunoprecipitated and immunoblotted with anti-HA antibody (fourth panel) and with anti-Xpress antibody (fifth panel). (D) BOSC23 cells were transfected with 2 μg of plasmids with the indicated combinations. At 72 h after transfection, cell lysates were immunoprecipitated with anti-Jun N-terminal kinase (JNK) antibody and the resultant immunoprecipitates were tested for JNK activity (top panel). WCLs were immunoprecipitated and immunoblotted with anti-JNK antibody (second panel). WCLs were also immunoblotted with the indicated antibodies to show the expression (bottom two panels). IP, immunoprecipitation; WB, western blot.
Next, the catalytic ability of ASK1 was examined (Fig 5C). Transfection of both ASK1 and ASK1(3M) enhanced self-phosphorylation and also phosphorylation of MKK6 and SEK1. The kinase activity was attenuated significantly by the coexpression of SUMO-1 and SUMO-1(ΔC6). However, ASK1(3M) kinase activity was not suppressed significantly by either SUMO-1 or SUMO-1(ΔC6). The kinase activity of ASK1 was significantly increased by SUMO-1 depletion, but not by scRNA.
This study examined whether the interaction between ASK1 and SUMO-1 influenced JNK signalling. As shown in Fig 5D, JNK activation was enhanced by transfections of ASK1, ASK1(3M) and siRNA–SUMO-1, but not by scRNA. ASK1-mediated JNK activation was significantly attenuated by the coexpression of SUMO-1(ΔC6) and SUMO-1. However, ASK1(3M)-mediated JNK activation was not suppressed efficiently by either SUMO-1 or SUMO-1(ΔC6).
Collectively, our data show that SUMO-1 suppresses ASK1 oligomerization and subsequently inhibits the catalytic function of ASK1. The negative regulation of ASK1 activation by SUMO-1 occurs independently of the covalent modification of ASK1.
ASK1-mediated cell death was inhibited by SUMO-1
As ASK1 can initiate cell death, we questioned whether SUMO-1 protected cells from ASK1-mediated death. Transfection of both ASK1 and ASK1(3M) enhanced cell death (Fig 6). Both SUMO-1 and SUMO-1(ΔC6) were able to suppress ASK1-mediated cell death. However, both SUMO-1 and SUMO-1(ΔC6) failed to suppress ASK1(3M)-mediated apoptosis. siRNA–SUMO-1 transfection potentiated cell death, indicating that SUMO-1 depletion relieved ASK1 from suppressive regulation by SUMO-1.
Figure 6.

Small ubiquitin-related modifier-1 suppresses cell death mediated by apoptosis signal-regulating kinase 1. BOSC23 cells were transfected with 2 μg of plasmids with or without short interfering RNA (siRNA)–small ubiquitin-related modifier-1 (SUMO-1) with the indicated combinations. At 72 h after transfection, cells were fixed and stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI). DAPIstained nuclei of green fluorescent protein (GFP)-positive cells were examined for apoptotic morphology by fluorescence microscopy and the percentages of apoptotic cells in five randomly chosen fields were determined. Data are mean±s.d. of triplicates from one experiment that is representative of three independent experiments (top panel). Whole-cell lysates (WCLs) were also immunoblotted with the indicated antibodies to show the expression. WB, western blot.
Discussion
This study presents a novel function of SUMO-1, which is mediated by interaction with the nonconjugated form of ASK1. The ASK1–SUMO-1 interaction was specific because substitutions of the three ASK1 lysines in the ψKXE motifs impaired the binding. However, substitutions of the ASK1 lysines did not compromise the interaction with Ubc9, indicating that the lysines in the ψKXE motifs have specific roles in ASK1–SUMO-1 interaction. The ASK1–SUMO-1 interaction inhibited the oligomerization of ASK1 and phosphorylation of downstream targets, resulting in the suppression of ASK1-mediated apoptotic cell death.
Inhibition of oligomerization by SUMO-1 is not unique to ASK1. Sumoylation of translocation E26 leukemia (TEL) protein inhibits oligomerization and disrupts nuclear body formation (Zhang et al, 2004a). However, ASK1 is different from TEL in that ASK1 does not require sumoylation. There are proteins that are regulated by SUMO-1 by physical interactions without covalent modifications. SUMO-1 inhibits homologous recombination by RAD51 independently of conjugation, and prevents Fas- and TNFR-mediated apoptosis without conjugating death receptors. Therefore, ASK1 can be a new addition to the proteins that are regulated by SUMO-1 without covalent modification. In addition, ASK1 does not have the known SUMO-1 interaction motif, hhXSXS/Taaa (where h represents a hydrophobic residue and a an acidic residue), which is observed in some sumoylated proteins (Minty et al, 2000). Thus, diverse modes seem to exist for SUMO-1 interaction.
The ASK1 lysines in ψKXE do not seem to affect the enzymatic function of ASK1. ASK1(3M) retained its self-oligomerization ability as well as autophosphorylation potential and successfully phosphorylated target proteins. ASK1-mediated apoptotic potential was also sustained. The fact that three lysines outside the kinase domain can downregulate ASK1-mediated signalling suggests that domains outside the kinase domain might have intramolecular regulatory roles.
In the case of RanGAP1, a well-known sumoylation target, the ψKXE motif was required for the establishment of Ubc9–RanGAP1 interaction as well as for the sumoylation of RanGAP1 (Sampson et al, 2001). The lysine acceptor in the ψKXE motif is not essential for the ASK1–Ubc9 interaction. The dissimilarity in requiring the ψKXE motif for Ubc9 interaction with RanGAP1 but not for that with ASK1 might be owing to differences in the amino-acid sequences surrounding the ψKXE motif. The apparent uncoupling between Ubc9 binding and SUMO-1 binding in the case of ASK1 might provide some insight into failure of the covalent modification. It is tempting to speculate that the physical proximity between the Ubc9 interaction site and the sumoylation site is required for Ubc9 to catalyse the covalent modification. In the case of RanGAP1, the Ubc9 interaction site and the sumoylation site are in such close proximity that changes in the ψKXE motif would affect the interaction with Ubc9. In the case of ASK1, however, the Ubc9 interaction site and the ψKXE motif might not be close enough for this effect.
Our data clearly show that SUMO-1 downregulates c-Jun activity. However, it is not clear whether SUMO-1 modulates the affinity to DNA or the interaction with transcription cofactors. Phosphorylation of c-Jun inhibits sumoylation and as a result, SUMO-1 and c-Jun seem to regulate mutually. A reciprocal regulation has also been observed in the case of Elk-1 (Yang et al, 2003). Phosphorylation of Elk-1 prevents sumoylation, and SUMO-1 modification represses Elk-1 activation. Thus, important pathways such as MAPK pathways seem to be carefully counterbalanced between two different mechanisms—activation by phosphorylation and repression by sumoylation. Moreover, this study shows the presence of another upstream target point regulated by SUMO-1 in c-Jun activation signalling. It is worthwhile to speculate on the benefit of having more than one target in the same signalling pathway. Redundant downregulation would guarantee the fidelity of the c-Jun activation further.
Methods
Cell culture and transfections. BOSC23 cells were grown at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Jeil Biotechservices Inc., Daegu, Korea) supplemented with 10% fetal bovine serum (FBS) and antibiotics (Invitrogen, San Diego, CA, USA). BOSC23 cells (1 × 106) were transfected with the indicated plasmids using the calcium phosphate method or the Lipofectamine transfection reagent (Invitrogen).
Plasmids and mutagenesis. The ASK1(3M) mutant was generated using a QuikChange kit (Stratagene, La Jolla, CA, USA). Constructions of Ubc9 and SUMO-1 vectors were reported previously (Ryu et al, 2000; Hwang et al, 2002). pcDNA3-HAsUMO-1 was kindly provided by Dr R.T. Hay (St Andrews University, St Andrews, UK). The SUMO-1(ΔC6) (amino acids 1–95) was amplified by PCR and subcloned into the pcDNA-MaxA and pGEX-4T-1 plasmids. The siRNA–SUMO-1 used in this study was reported previously (Kishi et al, 2003).
Antibodies. Mouse anti-Xpress (Invitrogen), mouse anti-Flag (M2) and mouse anti-β-tubulin antibodies and horseradish peroxidase-conjugated antibodies against rabbit IgG and mouse IgG were purchased from Sigma (St Louis, MO, USA). Rabbit anti-HA, mouse anti-ASK1 and goat anti-Ubc9 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-JNK (PharMingen, San Diego, CA, USA) and rabbit anti-SUMO-1 (LabFrontier Co., Seoul, Korea) antibodies were also purchased.
In vitro binding and co-immunoprecipitation analysis. 35S-labelled ASK1 and ASK1(3M) proteins were made with the in vitro TNT system (Promega, Madison, MD, USA). The expression and purification of GST fusion proteins and GST pull-down assays were carried out as described previously (Ryu et al, 2000). For co-immunoprecipitation experiments, lysates were subjected to immunoprecipitation by a 2 h incubation at 4°C with appropriate antibodies and then by a 2 h incubation at 4°C in the presence of protein A/Gsepharose beads (Santa Cruz Biotechnology). The resulting immunoprecipitates were subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and analysed by immunoblotting with the indicated antibodies.
Immunocomplex kinase assay. Cells were washed with phosphate-buffered saline (PBS) and lysed in the mammalian lysis buffer (Park et al, 2004). Supernatants containing 200 μg of protein were subjected to immunoprecipitation by a 2 h incubation at 4°C with appropriate antibodies. Immune complexes were recovered with the addition of protein A/Gsepharose beads. The beads were washed three times with lysis buffer and once with kinase buffer (Cho et al, 2004) and resuspended in 50 μl of the same kinase buffer. The beads were then incubated with GST–c-Jun, GST–MKK6, GST–SEK1 and 10 μCi [γ-32P]ATP with 20 μM cold ATP for 30 min at 30°C. Phosphorylated proteins were analysed by SDS–PAGE and autoradiographed.
Apoptotic cell death. Cultured cells were transfected with pEGFP-C2 (Clontech, Palo Alto, CA, USA) and indicated plasmids. At 72 h after transfection, cells were washed twice with PBS, fixed with 4% formaldehyde and stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes, Eugene, OR, USA). The DAPI-stained nuclei in green fluorescent protein (GFP)-positive cells were examined for apoptotic morphology by fluorescence microscopy (Bio-Red, Richmond, CA, USA).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures
Acknowledgments
This work was supported by grant number FG04-21-03 of 21C Frontier Functional Human Genome Project from MOST of Korea.
References
- Chen J, Fujii K, Zhang L, Roberts T, Fu H (2001) Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK–ERK independent mechanism. Proc Natl Acad Sci USA 98: 7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Ko HM, Kim JM, Lee JA, Park JE, Jang MS, Park SG, Lee DOH, Ryu SE, Park BC (2004) Positive regulation of apoptosis signal-regulating kinase 1 by hD53L1. J Biol Chem 279: 16050–16056 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Gill G (2004) SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev 18: 2046–2059 [DOI] [PubMed] [Google Scholar]
- Goldman EH, Chen L, Fu H (2004) Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem 279: 10442–10449 [DOI] [PubMed] [Google Scholar]
- Hwang IS, Jung YS, Kim E (2002) Interaction of ALG-2 with ASK1 influences ASK1 localization and subsequent JNK activation. FEBS Lett 529: 183–187 [DOI] [PubMed] [Google Scholar]
- Johnson ES, Blobel G (1999) Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol 147: 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Choi CY, Kim Y (1999) Covalent modification of the homeodomain-interacting protein kinase 2 (HIPK2) by the ubiquitin-like protein SUMO-1. Proc Natl Acad Sci USA 96: 12350–12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi A, Nakamura T, Nishio Y, Maegawa H, Kashiwagi A (2003) Sumoylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am J Physiol Endocrinol Metab 284: E830–E840 [DOI] [PubMed] [Google Scholar]
- Li W, Hesabi B, Babbo A, Pacione C, Liu J, Chen DJ, Nickoloff JA, Shen Z (2000) Regulation of doublestrand break-induced mammalian homologous recombination by UBL1, a RAD51-interacting protein. Nucleic Acids Res 28: 1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis MJ, Wu J, Blobel G (1998) SUMO-1 modification and its role in targeting the Pan GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol 140: 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A, Dumont X, Kaghad M, Caput D (2000) Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J Biol Chem 275: 36316–36323 [DOI] [PubMed] [Google Scholar]
- Mishra RK, Jatiani SS, Kumar A, Simhadri VR, Hosur RV, Mittal R (2004) Dynamin interacts with members of the sumoylation machinery. J Biol Chem 279: 31445–31454 [DOI] [PubMed] [Google Scholar]
- Muller S, Hoege C, Pyrowolakis G, Jentsch S (2001) SUMO, ubiquitin's mysterious cousin. Nat Rev Mol Cell Biol 2: 202–210 [DOI] [PubMed] [Google Scholar]
- Muller S, Ledl A, Schmidt D (2004) SUMO: a regulator of gene expression and genome integrity. Oncogene 23: 1998–2008 [DOI] [PubMed] [Google Scholar]
- Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei CF, Chang HM, Yeh ET (1996) Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol 157: 4277–4281 [PubMed] [Google Scholar]
- Park MY, Jang HD, Lee SY, Lee KJ, Kim E (2004) Fas-associated factor-1 inhibits nuclear factor-κB (NF-κB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-κB. J Biol Chem 279: 2544–2549 [DOI] [PubMed] [Google Scholar]
- Ryu SW, Chae SK, Kim E (2000) Interaction of Daxx, a Fas binding proteins, with sentrin and Ubc9. Biochem Biophys Res Commun 279: 6–10 [DOI] [PubMed] [Google Scholar]
- Sampson DA, Wang M, Matunis MJ (2001) The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276: 21664–21669 [DOI] [PubMed] [Google Scholar]
- Yang SH, Jaffray E, Hay RT, Sharrocks AD (2003) Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol Cell 12: 63–74 [DOI] [PubMed] [Google Scholar]
- Zhang H, Smolen GA, Palmer R, Christoforou A, van den Heuvel S, Haber DA (2004a) SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans Polycomb group protein SOP-2. Nat Genet 36: 507–511 [DOI] [PubMed] [Google Scholar]
- Zhang L, Fogg DK, Waisman DM (2004b) RNA interference-mediated silencing of the S100A10 gene attenuates plasmin generation and invasiveness of Colo 222 colorectal cancer cells. J Biol Chem 279: 2053–2062 [DOI] [PubMed] [Google Scholar]
- Zhong S, Salomoni P, Pandolfi PP (2000) The transcriptional role of PML and the nuclear body. Nat Cell Biol 2: E85–E90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


