Abstract
The TVB receptor for subgroup B, D, and E avian sarcoma and leukosis viruses (ASLVs) is a tumor necrosis factor receptor-related protein that is most closely related to the TRAIL receptors. Here we show that the major subgroup B viral interaction determinants of TVB are contained within a linear 15-amino-acid peptide derived from the N-terminal region of the receptor. Moreover, this peptide was sufficient not only for binding to ASLV-B but also for activating viral entry into mammalian cells that lacked the cognate viral receptor. Peptide-dependent viral entry was blocked in the presence of bafilomycin A1, indicating that virions can be trafficked to an acidic endosomal fusion compartment without the need for physical attachment of the viral receptor to a cellular membrane.
Retrovirus entry into a cell is mediated by interactions between the viral envelope protein (Env) and cellular receptors. Env proteins are trimers of heterodimers composed of surface (SU) and transmembrane (TM) subunits. SU contains determinants which are required for receptor interaction. TM contains four motifs required for virus-cell membrane fusion: a hydrophobic fusion peptide located at or near its N terminus, two extracellular heptad-repeat regions (an N-helix and a C-helix) with the propensity to form coiled-coil structures, and a membrane-spanning domain (21). For most retroviruses, membrane fusion is thought to be driven by receptor/coreceptor-induced conformational changes in Env. These changes lead to exposure of the fusion peptide so that it can insert into the target membrane (21). Subsequently, TM undergoes additional conformational changes that lead to formation of a six-helix bundle at the time of membrane fusion (21, 33). Structural similarities between the fusion proteins of retroviruses and those of filoviruses, orthomyxoviruses, and paramyxoviruses suggest a common entry mechanism (21), although the steps that lead to their fusion activation remain incompletely defined.
Avian sarcoma and leukosis viruses (ASLV) are alpharetroviruses that provide an experimentally tractable model system for studying the mechanism of retroviral entry. ASLV are classified into 10 different receptor usage subgroups (designated A through J) (28). Cellular receptors for four ASLV subgroups have been cloned: the TVA receptor for ASLV-A (7, 8, 56) and the TVB receptors for ASLV-B, ASLV-D, and ASLV-E (2, 3, 12, 46). There are two functional alleles of chicken tvb (tvbs1 and tvbs3) that encode receptors for ASLV-B, -D, and -E and for ASLV-B and -D, respectively (3, 12). The turkey homolog of this gene (tvbt) encodes an ASLV-E-specific receptor (2).
TVA is a member of the low-density-lipoprotein receptor family (8), whereas TVB is a tumor necrosis factor receptor (TNFR)-related death receptor that is most likely the avian homolog of a TRAIL receptor (11, 12, 14, 31, 37, 43, 44, 50). Thus, TVB may contribute to the virus-associated cell killing events associated with subgroup B and D ASLV infections, even though subgroup E viruses, which use the same cellular receptor, are generally noncytopathic (1-3, 12, 20).
TVA and TVB are simple type I transmembrane proteins that appear to be sufficient for conferring susceptibility to viral infection. These features have been exploited to generate soluble forms of these receptors that retain their abilities to bind to and activate ASLV Env for fusion (6, 10, 15, 16, 25, 47-49). A model that invokes receptor binding as sufficient to promote ASLV Env-dependent membrane fusion has been proposed (16-18, 23-25). However, we have recently obtained multiple lines of evidence that ASLV entry instead requires an initial receptor-priming step followed by a low-pH activation step, indicating that the virus enters into cells from an acidic endosomal compartment (discussed in detail in reference 34).
Previous studies of TVB had shown that the major ASLV-B interaction determinants reside within its N-terminal region (amino acid residues 1 to 101) (1). Furthermore, the subgroup B Env-binding region of TVB seemed to be nonconformational in nature, since it was not affected by mutagenesis of the first four cysteine residues or by treatments leading to reduction and denaturation of the receptor (1). Here we show that the major ASLV-B interaction determinants of TVB are contained on a short linear peptide which binds directly to the virus, allowing viral entry into receptor-negative cell lines. Peptide-dependent viral entry was blocked in the presence of a v-type H+-ATPase inhibitor, indicating that peptide-associated virions traffic to an acidic endosomal fusion compartment.
MATERIALS AND METHODS
Cell lines, viruses, and immunoadhesins.
Human 293 cells, mouse NIH 3T3 and B16 cells, monkey COS-7 cells, and pig PAE cells were obtained from the American Type Culture Collection. Chicken DF1 cells and primary chicken embryo fibroblast (C/ABE) cells have been described previously (4, 26). 293(2.1) cells were stably transfected with plasmid pPUR (Clontech) and with a plasmid vector containing a synthetic quail TVA cDNA clone (9). 293 (S1-5) cells were stably transfected with pPUR and with a plasmid encoding TVBS1(ΔDD), a FLAG epitope-tagged version of the TVBS1 receptor without the cytoplasmic death domain (3). Both cell types were grown in medium containing 1 μg of puromycin/ml. The RCASBP(A)-EFGP and RCASBP(B)-EGFP viruses encoding enhanced green fluorescent protein (EGFP) were produced from cultures of chronically infected DF1 cells as described previously (11, 47). The SUA-rabbit immunoglobulin G (rIgG), SUB-rIgG, and SUE-rIgG fusion proteins were produced in the extracellular supernatants of transiently transfected human 293 cells as described previously (2, 12, 59).
Enzyme-linked immunosorbent assays (ELISAs).
Biotinylated peptides were purchased either from the BCMP Biopolymers Facility at Harvard Medical School or from Research Genetics Corp., resuspended in vacuum-degassed distilled water, and stored at −80°C. One hundred nanograms of each biotinylated peptide was loaded onto individual wells of a 96-well plate coated with streptavidin (Pierce) in 100 μl of wash buffer (25 mM Tris, 150 mM NaCl, 0.1% bovine serum albumin, 0.05% Tween 20 [pH 7.2]) for 2 h at room temperature. The plate was then washed three times with wash buffer, and 100 μl of extracellular supernatant containing the different SU-rIgG fusion proteins was added for 1 h at room temperature. The plate was washed as before, and 100 μl of a horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antibody (1:1,000 dilution; Amersham) was added for 30 min. The plate was again washed as before, and 100 μl of tetramethyl benzidine-peroxidase (Pierce) was added for 15 min according to the manufacturer's instructions. Then 100 μl of 2 M H2SO4 was added, and the absorbance of each sample at 450 nm was measured with a Spectramax instrument (Molecular Devices).
Flow cytometry.
Cells were prepared for flow cytometry as described previously (59). Uninfected DF1 cells and DF1 cells chronically infected with RCASBP(B)-EGFP were incubated for 30 min at 4°C with increasing amounts (0.1 nM to 10 μM concentrations) of biotinylated TVB32-46 (Research Genetics Corp.) in a 1-ml volume of phosphate-buffered saline (PBS) supplemented with 1% bovine calf serum (BPBS). Cells were then washed with 1 ml of BPBS and incubated for 30 min at 4°C with 4 μg of streptavidin-allophycocyanin (SA-APC) (Molecular Probes)/ml in 0.5 ml and then with 7 μM propidium iodide to identify dead cells. APC fluorescence was then quantified by using a FACScan flow cytometer with Cell Quest software (both from Becton Dickinson). On average, 104 cells were analyzed per sample.
Infections.
RCASBP(B)-EGFP was incubated for 1 h at 4°C with cell culture medium containing increasing amounts (100 pM to 10 μM concentrations) of biotinylated TVB32-46 (Research Genetics). A 1-ml aliquot of the virus-peptide complex was then added to each well (containing approximately 105 293 cells) of a 6-well plate, corresponding to a multiplicity of infection of 3 GFP-transducing units as determined by virus infection of 293 (S1-5) cells. The plates were then either spun at 1,000 × g for 90 min at 23°C (i.e., spinoculated [described in reference 36]) and then incubated at 37°C or instead incubated at 37°C without a centrifugation step. Approximately 18 h later, 1 ml of fresh medium was added to each well; 56 h later, the cells were trypsinized and fixed in 1% formaldehyde in PBS, and infected cells expressing EGFP were detected by using a FACScaliber flow cytometer (Becton Dickinson).
TVA/B receptors.
The TVA/TVB (TVA/B) chimera, encoded by plasmid pDK007, was created by PCR-based insertional mutagenesis of a synthetic quail TVA gene (9), subcloned between the EcoRI and XbaI sites of expression plasmid pBK-CMV (Stratagene). A DNA fragment encoding the TVB32-46 peptide was inserted at the HindIII site between the 27th and 28th codons of the TVA open reading frame (9). The nucleotide sequence of the insert is AAGCTTGGACCGCTCGGACCTCCAGAAGCCAGATCTCTACCGGCGGAAGTCACTCGAG (HindIII and XhoI sites are underlined). The TVA/B* construct was generated in plasmid pDK013 by replacing this HindIII-XhoI fragment with annealed double-stranded DNA oligonucleotides encoding a mutant TVB32-46 peptide sequence with the L36V, Q37L, and L41P substitutions incorporated.
Approximately 2 × 106 293 cells were transfected by the calcium phosphate precipitation method (53) with 1.5 μg of plasmid pPUR (Clontech) and with 15 μg of either plasmid pDK007 or plasmid pDK013. After 72 h, the cells were incubated with medium containing 1 μg of puromycin/ml, and single cell clones were expanded and analyzed for TVA/B and TVA/B* expression by a flow cytometric assay that uses SUA-rIgG (59). Cell lines DK007.6 and DK013.1 were judged to have equivalent levels of TVA/B and TVA/B* proteins, respectively. These cells were subjected to flow cytometric analysis with SUB-rIgG and SUA-rIgG and to challenge with the RCASBP(A)-EGFP and RCASBP(B)-EGFP viruses as described above.
Inhibition of viral infection with bafilomycin A1.
Experiments with bafilomycin A1 were performed by spinoculation (a method adapted from reference 36) of RCASBP(B)-EGFP preloaded with TVB32-46 onto the surfaces of human 293 cells as described above. The only difference was that the cells were incubated in a medium containing 200 nM bafilomycin A1 30 min before viral challenge and for the duration of the experiment thereafter, until the DNA was harvested for quantitative measurements using a real-time PCR assay that has been described elsewhere (34).
RESULTS
Identification of a short peptide which binds subgroup B ASLV Env.
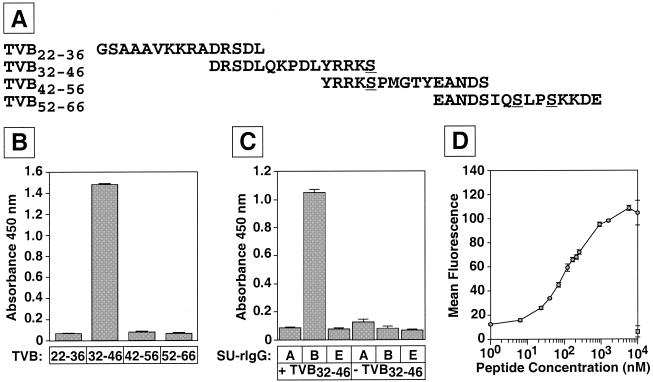
To test the hypothesis that the major ASLV-B interaction determinants may reside within a linear peptide located near the amino terminus of TVBS1 and TVBS3, a series of four 15-amino-acid peptides, each biotinylated at its N terminus, was synthesized (Fig. 1A). Together, these peptides encompassed the first 45 amino acids of the mature form of the chicken TVB receptor. To prevent any problems associated with intermolecular disulfide bonding, residues corresponding to Cys-46, Cys-59, and Cys-62 were changed to serines (Fig. 1A), since it was already known that these amino acid substitutions do not affect ASLV-B entry via the transmembrane TVBS1 receptor (3).
FIG. 1.
A 15-amino-acid peptide derived from the TVB receptor binds to ASLV-B SU. (A) Four overlapping biotinylated synthetic peptides derived from the first 45 residues of TVBS1 and TVBS3 (3). Residues corresponding to Cys-46, Cys-59, and Cys-62 were changed to serines (underlined). (B) TVB32-46 binds to an ASLV-B SU-immunoglobulin fusion protein. An ELISA was performed with the biotinylated peptides bound to streptavidin-coated plates and a subgroup B ASLV SU-immunoglobulin fusion protein, SUB-rIgG (12). The bound SUB-rIgG was detected by using an HRP-conjugated secondary antibody and a colorimetric substrate. (C) TVB32-46 binds specifically to SUB-rIgG. The ELISA was performed as for panel B with wells that were either coated (+TVB32-46) or not (−TVB32-46) with peptide and also with equal amounts of subgroup A- and E-specific SU-immunoglobulin proteins (SUA-rIgG and SUE-rIgG, respectively). (D) TVB32-46 binds to native ASLV-B Env expressed at the surfaces of infected cells. Chicken DF1 cells chronically infected with a subgroup B virus encoding EGFP [RCASBP(B)-EGFP] were incubated with increasing amounts of biotinylated TVB32-46 and with SA-APC (circle). For control purposes, uninfected DF1 cells were incubated with the maximal amount (a 10 μM concentration) of peptide used (square). The cell-associated APC fluorescent signal was quantified by flow cytometry. Data in panels B, C, and D are means and standard deviations (error bars) from at least two independent experiments that were performed in triplicate.
Each peptide was tested for its ability to bind to a subgroup B ASLV SU-immunoglobulin fusion protein (an immunoadhesin, designated SUB-rIgG [12]) in an ELISA. The biotinylated peptides were loaded into individual wells of a streptavidin-coated 96-well plate and exposed to SUB-rIgG. After removal of unbound immunoadhesin, the bound SUB-rIgG was detected by using an HRP-linked anti-rabbit secondary antibody and a colorimetric substrate. This analysis revealed that one peptide, designated TVB32-46, bound specifically to SUB-rIgG (Fig. 1B). Furthermore, this binding interaction was specific for ASLV-B, since the peptide did not bind to either subgroup A- or subgroup E-specific ASLV SU-rIgG proteins (Fig. 1C).
To confirm that the peptide was capable of binding to the native ASLV-B Env trimer (22), biotinylated TVB32-46 was incubated with avian cells chronically infected with a subgroup B ASLV vector. Peptide that was bound to the cell surface Env was detected by use of SA-APC. This experiment showed that TVB32-46 bound to cell surface ASLV-B Env in a dose-dependent manner, with maximal binding achieved in the low micromolar range (Fig. 1D). By contrast, no binding of TVB32-46 to uninfected DF1 cells was detected (Fig. 1D).
TVB32-46 binding activates ASLV-B for fusion with receptor-negative cells.
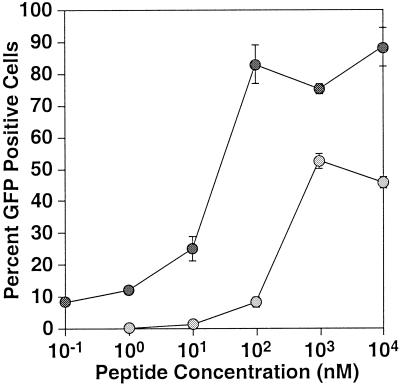
To test whether TVB32-46 binding is capable of activating ASLV-B for entry, the biotinylated peptide was preloaded onto an ASLV-B virus produced from vector RCASBP(B)-EGFP. These peptide-loaded viruses were then added to human 293 cells and assayed for infection several days later by monitoring of EGFP expression. Strikingly, TVB32-46 efficiently promoted ASLV-B entry into 293 cells at concentrations ranging from 100 nM to 1 μM (Fig. 2). The maximal level of infection achieved (Fig. 2) represented 25% of that seen when the same amount of native virions was used to infect transfected human 293 cells expressing TVB. Peptide-mediated infection was significantly enhanced when virions were gently centrifuged (spinoculated [5, 16, 27, 30, 36]) onto cell surfaces. TVB32-46 also mediated subgroup B viral entry into a variety of other receptor-negative avian and mammalian cell types (ASLV-B-resistant chicken embryo fibroblasts [C/ABE cells] and NIH 3T3, COS-7, B16, and PAE cells) (data not shown). Similar results were obtained with a nonbiotinylated version of TVB32-46 (data not shown).
FIG. 2.
TVB32-46 allows ASLV-B infection of receptor-negative cells. TVB32-46 was mixed with the subgroup B virus RCASBP(B)-EGFP, and the virus-peptide complex was then added to cells with (dark shaded circles) or without (light shaded circles) a gentle centrifugation (spinoculation) step. Infection was then quantified by flow cytometric analysis to measure the number of infected (EGFP-positive) cells. Data are means and standard deviations (error bars) from at least four independent experiments, each performed in triplicate. These experiments were performed at a multiplicity of infection of 3 GFP-transducing units (measured by infecting TVB-expressing human 293 cells with the same amount of native virions) as described in Materials and Methods.
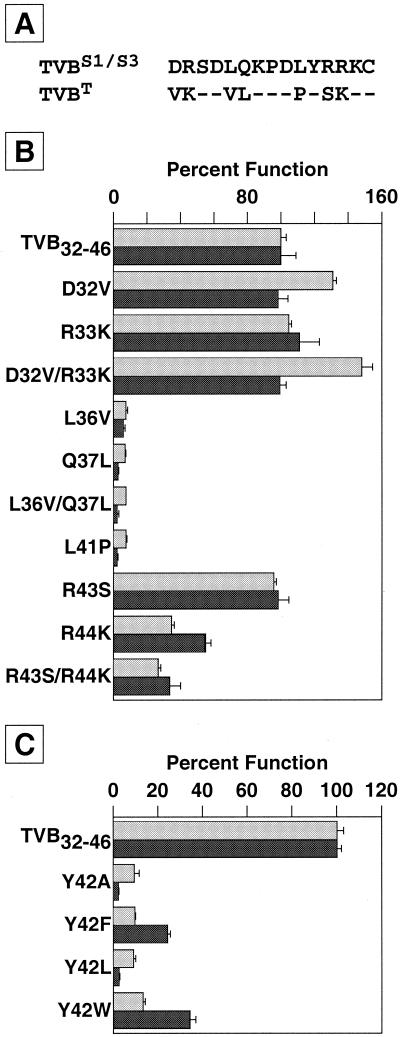
Identification of four residues of TVB32-46 that are important for subgroup B viral interaction.
The chicken TVBS1 and TVBS3 proteins are ASLV-B receptors, whereas the turkey TVBT protein is not (3). The chicken proteins differ from TVBT at 7 of the 15 amino acid positions encompassed by residues TVB32-46 (Fig. 3A), indicating that 1 or more of these residues may be important for receptor function. To test this idea, a panel of altered peptides was synthesized and tested for the ability to bind to SUB-rIgG and to activate viral entry. Replacement of one of three residues of TVB32-46 with the corresponding TVBT residue (L36V, Q37L, or L41P) almost completely abolished the ability of the peptide to serve as an ASLV-B receptor (Fig. 3B). Also, a peptide containing all three of these amino acid substitutions failed to bind to SUB-rIgG and did not promote viral entry (data not shown). By contrast, the other mutations tested (D32V, R33K, D32V R33K, R43S, R44K, and R43S R44K) had very little impact on peptide function (Fig. 3B).
FIG. 3.
Identification of four functionally important residues of TVB32-46. (A) The amino acid sequences of the chicken TVBS1 and TVBS3 receptors and of the turkey TVBT protein are shown aligned over the region represented by TVB32-46. Dashes represent conserved residues. (B) Residues Leu-36, Gln-37, and Leu-41 are important for TVB32-46 function. A set of altered biotinylated peptides was generated in which residues that were specific to TVBT were used to replace the corresponding residues in the TVB32-46 peptide. These altered peptides were tested for binding to SUB-rIgG (light shaded bars) and for the ability to mediate viral infection (dark shaded bars) as described in the legends to Fig. 1B and C and Fig. 2. (C) Residue Tyr-42 is important for TVB32-46 function. An additional set of peptides was also synthesized in which residue Tyr-42 was replaced with other amino acids, and these peptides were tested as in the experiment for which results are shown in panel B. In panels B and C, 100% infection represents a multiplicity of infection of 0.36 (B) or 0.6 (C) GFP-transducing units (measured from the level of infection achieved in the presence of the wild-type TVB32-46 peptide). Data are means and standard deviations (error bars) from at least three independent experiments that were performed in triplicate.
It was also noteworthy that TVB32-46 contains a single aromatic residue (Tyr-42), since an aromatic residue is an important viral-interaction determinant of other retroviral receptors, including CD4 for human immunodeficiency virus type 1 (HIV-1) and HIV-2, mCAT-1 for ecotropic murine leukemia virus (MLV), and TVA for subgroup A ASLV (32, 41, 42, 54, 55, 57-59). Indeed, an aromatic residue located at this position of TVB32-46 is important for subgroup B receptor function, since altered peptides containing a Y42F or Y42W substitution partially supported ASLV-B infection whereas those bearing a Y42A or Y42L substitution did not (Fig. 3C). In conclusion, these experiments have identified four residues (Leu-36, Gln-37, Leu-41, and Tyr-42) that are important for subgroup B receptor activity.
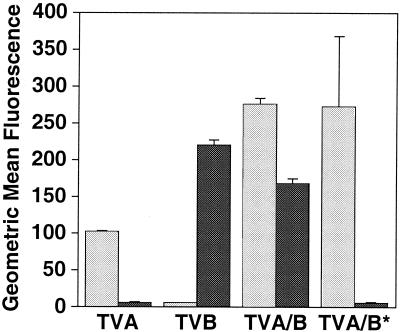
A recombinant TVA protein containing TVB32-46 mediates ASLV-B entry.
The activity of TVB32-46 was next tested in the context of a recombinant membrane-associated protein designated TVA/B. TVA/B contains the TVB32-46 peptide sequence inserted between the 8th and 9th amino acids of the mature TVA protein, at a site that has previously been shown to accommodate an epitope tag without affecting subgroup A viral receptor function (59). In contrast to TVA, which served only as a subgroup A viral receptor, and to TVB, which served only as a subgroup B viral receptor, TVA/B was a receptor for both viral subgroups (Fig. 4 and Table 1). By contrast, an altered version of this protein, TVA/B*, bearing the function-perturbing L36V Q37L L41P substitutions was capable only of supporting ASLV-A entry (Fig. 4 and Table 1).
FIG. 4.
A chimeric TVA protein containing TVB32-46 binds to both ASLV-A and ASLV-B SU. Chimeric TVA receptors TVA/B and TVA/B*, containing, respectively, either the wild-type TVB32-46 peptide or a triply substituted (L36V Q37L L41P) version, were generated. Human 293 cells that expressed equivalent amounts of either TVA/B or TVA/B* were incubated with either SUA-rIgG (light shaded bars) or SUB-rIgG (dark shaded bars) and a fluorescein isothiocyanate-conjugated secondary antibody and were subjected to flow cytometry. Data are means and standard deviations (error bars) from at least two independent experiments that were performed in triplicate.
TABLE 1.
Cells expressing TVA/B are susceptible to infection by both subgroups A and B of ASLV
| Receptor | Multiplicity of infection
|
|
|---|---|---|
| ASLV-A | ASLV-B | |
| TVA | 1.97 | ≤0.003 |
| TVB | ≤0.003 | 1.9 |
| TVA/B | 1.24 | 0.42 |
| TVA/B∗ | 2.53 | ≤0.003 |
TVB32-46 supports low-pH-dependent viral entry.
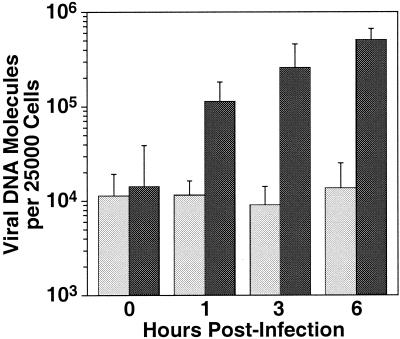
Recent work from our laboratory has demonstrated that ASLV entry involves receptor priming of the viral glycoprotein followed by low-pH-activated membrane fusion (34). To test whether there was a similar requirement for low pH during TVB32-46-mediated entry, infections were performed in the presence of 200 nM bafilomycin A1, a vacuolar H+-ATPase inhibitor (19, 34). Previously, we showed that this concentration of inhibitor could effectively block the entry of retroviral particles bearing ASLV Env, but not MLV Env (34). Therefore, this ASLV Env-specific block to viral entry cannot be due to any nonspecific toxic effect of the inhibitor. At different time points after viral challenge, DNA was harvested for real-time PCR quantitation of viral reverse transcription products. In the absence of bafilomycin A1, the number of viral DNA molecules increased 50-fold over the number associated with input virions (104 copies per 25,000 cells) (Fig. 5). This increase was inhibited in the presence of bafilomycin A1 (Fig. 5), consistent with a low-pH requirement for TVB32-46-dependent entry.
FIG. 5.
TVB32-46-dependent viral entry is blocked by bafilomycin A1. Infections were performed essentially as described in the legend to Fig. 2 except that cells were incubated in the presence (light shaded bars) or absence (dark shaded bars) of 200 nM bafilomycin A1. DNA samples were then harvested at different time points after virus addition for real-time PCR determination of the number of reverse transcription products. Data presented are means and standard deviations (error bars) compiled from two independent experiments, each performed in triplicate.
DISCUSSION
In this study, we have found that a linear 15-amino-acid peptide representing amino acids 32 to 46 of the chicken TVB receptor is capable of binding to subgroup B ASLV Env and activating viral entry into receptor-negative cells by a low-pH-dependent mechanism. Additionally, we have defined four amino acid residues that are important for peptide function: three residues that are specific to the chicken TVBS1 and TVBS3 receptors as well as a tyrosine residue that is shared with the turkey TVBT receptor. Therefore, as for a number of other retroviral receptors including those for HIV-1 and HIV-2 (Phe-43 ([54]), ecotropic MLV (Tyr 235 [32, 55, 57]), and ASLV-A (Trp-48 [41, 42, 58, 59]), an aromatic residue is a critical determinant of TVB receptor function. These simple requirements for ASLV-B receptor function differ markedly from those for ASLV-E interaction, which seem to be conformational in nature (3).
Since the peptide-loaded viral complexes infect receptor-negative cells in a low-pH-dependent manner, these complexes are presumably trafficked to a low-pH endosomal compartment where virus-cell membrane fusion occurs. In all likelihood, these viruses are taken up into cells and trafficked to these sites after they interact with other cell surface-associated molecules. Previously it was shown that a soluble version of the entire extracellular domain of TVA is capable of binding to ASLV-A and activating viral entry (16). Although these results were used to argue that the receptor is sufficient to activate viral entry, our results suggest that TVA-loaded virions are probably also trafficked to a low-pH endosomal fusion compartment after interacting with some as yet undefined cell surface molecule(s). These cell surface interactions may not involve the viral envelope glycoprotein, since several retroviruses including ASLV and MLV are known to interact with cell surfaces in a manner that is independent of Env-receptor contact (35, 39, 40). The molecules involved in these interactions have not yet been identified, and the nature of the association between the virus-peptide complexes and receptor-negative cells is currently being investigated.
The TVB32-46 peptide is located within a region that is predicted to be conformationally flexible in the full-length TVB receptor: X-ray crystallographic analysis of the highly related DR5 protein revealed that the corresponding region upstream of cysteine-rich domain 1 (CRD1) is structurally disordered (29). This region is predicted to lie outside of the main ligand-binding region of the TVB receptor, which, by analogy with other TNFR-related receptors, is likely to be constituted by determinants located within the extracellular CRD2 and CRD3 regions (29). However, this region may overlap with the predicted pre-ligand assembly domain (PLAD) of this receptor. PLAD domains have been defined in regions overlapping CRD1 in TNFR-1 and Fas; they also exist in mammalian TRAIL receptors (13, 38, 45) and mediate oligomerization of TNFR-related death receptors into functional complexes prior to ligand binding. Thus, it is possible that binding of ASLV-B Env to the TVB receptor would disrupt the formation of PLAD-dependent oligomers, an event that might in turn influence the activity of the TVB receptor, perhaps leading to virus-induced cell death during the acute phase of infection (11, 12, 20, 51, 52). Understanding of the precise effect of viral infection on normal physiological TVB-associated signal transduction pathways awaits the cloning and characterization of the TVB ligand.
In summary, the identification of a linear peptide of the TVB receptor which can serve as a minimal receptor for subgroup B ASLV has provided new insights into the differences between the interactions of the TVB receptor with cytopathic subgroup B viruses and its interactions with noncytopathic subgroup E viruses. This peptide is now being used as a powerful tool to obtain an understanding of these pathogenic differences and to study critical aspects of the ASLV-B entry mechanism, including defining the receptor-binding region of Env, investigating the molecular nature of the receptor-primed state of the viral glycoprotein that exists prior to low-pH fusion activation, and understanding the minimal requirements for virus-cell membrane fusion.
Acknowledgments
We thank the members of the Young laboratory, especially Ken Bradley, for helpful discussions and assistance with reagents and Tiffany Brake for assistance with the initial ELISA experiments. We thank Ken Bradley, Richard Barnard, Ryan Larson, Shakti Narayan, Sara Klucking, and Matt Oberley for critical reading of the manuscript. We thank Kelly Wasmund and Markus Gross at Research Genetics Corp. for valuable assistance with peptide synthesis.
This work was supported by NIH grant CA70810 (to J.A.T.Y.). D.J.K. is supported by an NCI training grant (T32 CA09135).
REFERENCES
- 1.Adkins, H. B., S. C. Blacklow, and J. A. T. Young. 2001. Two functionally distinct forms of a retroviral receptor explain the nonreciprocal receptor interference among subgroup B, D, and E avian leukosis viruses. J. Virol. 75:3520-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, H. B., J. Brojatsch, J. Naughton, M. M. Rolls, J. M. Pesola, and J. A. T. Young. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. USA 94:11617-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins, H. B., J. Brojatsch, and J. A. Young. 2000. Identification and characterization of a shared TNFR-related receptor for subgroup B, D, and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J. Virol. 74:3572-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon, L. D., H. D. Hunt, and H. H. Cheng. 2000. A review of the development of chicken lines to resolve genes determining resistance to diseases. Poult. Sci. 79:1082-1093. [DOI] [PubMed] [Google Scholar]
- 5.Bahnson, A. B., J. T. Dunigan, B. E. Baysal, T. Mohney, R. W. Atchison, M. T. Nimgaonkar, E. D. Ball, and J. A. Barranger. 1995. Centrifugal enhancement of retroviral mediated gene transfer. J. Virol. Methods 54:131-143. [DOI] [PubMed] [Google Scholar]
- 6.Balliet, J. W., J. Berson, C. M. D'Cruz, J. Huang, J. Crane, J. M. Gilbert, and P. Bates. 1999. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates, P., L. Rong, H. E. Varmus, J. A. T. Young, and L. B. Crittenden. 1998. Genetic mapping of the cloned subgroup A avian sarcoma and leukosis virus receptor gene to the TVA locus. J. Virol. 72:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 9.Bélanger, C., K. Zingler, and J. A. T. Young. 1995. Importance of cysteines in the LDLR-related domain of the subgroup A avian leukosis and sarcoma virus receptor for viral entry. J. Virol. 69:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boerger, A. L., S. Snitkovsky, and J. A. Young. 1999. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc. Natl. Acad. Sci. USA 96:9867-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brojatsch, J., J. Naughton, H. B. Adkins, and J. A. T. Young. 2000. TVB receptors for cytopathic and noncytopathic subgroups of avian leukosis viruses are functional death receptors. J. Virol. 74:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 13.Chan, F. K., H. J. Chun, L. Zheng, R. M. Siegel, K. L. Bui, and M. J. Lenardo. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288:2351-2354. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary, P. M., M. Eby, A. Jasmin, A. Bookwalter, J. Murray, and L. Hood. 1997. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-κB pathway. Immunity 7:821-830. [DOI] [PubMed] [Google Scholar]
- 15.Connolly, L., K. Zingler, and J. A. T. Young. 1994. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J. Virol. 68:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damico, R., L. Rong, and P. Bates. 1999. Substitutions in the receptor-binding domain of the avian sarcoma and leukosis virus envelope uncouple receptor-triggered structural rearrangements in the surface and transmembrane subunits. J. Virol. 73:3087-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Duve, C., T. de Barsy, B. Poole, A. Trouet, P. Tulkens, and F. Van Hoof. 1974. Lysosomotropic agents. Biochem. Pharmacol. 23:2495-2531. [DOI] [PubMed] [Google Scholar]
- 20.Dorner, A. J., and J. M. Coffin. 1986. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell 45:365-374. [DOI] [PubMed] [Google Scholar]
- 21.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 22.Einfeld, D., and E. Hunter. 1988. Oligomeric structure of a prototype retrovirus glycoprotein. Proc. Natl. Acad. Sci. USA 85:8688-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert, J. M., D. Mason, and J. M. White. 1990. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J. Virol. 64:5106-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Himly, M., D. N. Foster, I. Bottoli, J. S. Iacovoni, and P. K. Vogt. 1998. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248:295-304. [DOI] [PubMed] [Google Scholar]
- 27.Ho, W. Z., R. Cherukuri, S. D. Ge, J. R. Cutilli, L. Song, S. Whitko, and S. D. Douglas. 1993. Centrifugal enhancement of human immunodeficiency virus type 1 infection and human cytomegalovirus gene expression in human primary monocyte/macrophages in vitro. J. Leukoc. Biol. 53:208-212. [DOI] [PubMed] [Google Scholar]
- 28.Hunter, E. 1997. Viral entry and receptors, p. 71-119. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 29.Hymowitz, S. G., H. W. Christinger, G. Fuh, M. Ultsch, M. O'Connell, R. F. Kelley, A. Ashkenazi, and A. M. de Vos. 1999. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol. Cell 4:563-571. [DOI] [PubMed] [Google Scholar]
- 30.Kotani, H., P. B. Newton III, S. Zhang, Y. L. Chiang, E. Otto, L. Weaver, R. M. Blaese, W. F. Anderson, and G. J. McGarrity. 1994. Improved methods of retroviral vector transduction and production for gene therapy. Hum. Gene Ther. 5:19-28. [DOI] [PubMed] [Google Scholar]
- 31.MacFarlane, M., M. Ahmad, S. M. Srinivasula, T. Fernandes-Alnemri, G. M. Cohen, and E. S. Alnemri. 1997. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 272:25417-25420. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra, S., A. G. Scott, T. Zavorotinskaya, and L. M. Albritton. 1996. Analysis of the murine ecotropic leukemia virus receptor reveals a common biochemical determinant on diverse cell surface receptors that is essential to retrovirus entry. J. Virol. 70:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 35.Notter, M. F., J. F. Leary, and P. C. Balduzzi. 1982. Adsorption of Rous sarcoma virus to genetically susceptible and resistant chicken cells studied by laser flow cytometry. J. Virol. 41:958-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan, G., K. O'Rourke, A. M. Chinnaiyan, R. Gentz, R. Ebner, J. Ni, and V. M. Dixit. 1997. The receptor for the cytotoxic ligand TRAIL. Science 276:111-113. [DOI] [PubMed] [Google Scholar]
- 38.Papoff, G., P. Hausler, A. Eramo, M. G. Pagano, G. Di Leve, A. Signore, and G. Ruberti. 1999. Identification and characterization of a ligand-independent oligomerization domain in the extracellular region of the CD95 death receptor. J. Biol. Chem. 274:38241-38250. [DOI] [PubMed] [Google Scholar]
- 39.Piraino, F. 1967. The mechanism of genetic resistance of chick embryo cells to infection by Rous sarcoma virus-Bryan strain (BS-RSV). Virology 32:700-707. [DOI] [PubMed] [Google Scholar]
- 40.Pizzato, M., S. A. Marlow, E. D. Blair, and Y. Takeuchi. 1999. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 73:8599-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong, L., K. Gendron, and P. Bates. 1998. Conversion of a human low-density lipoprotein receptor ligand binding repeat to a virus receptor: identification of residues important for ligand specificity. Proc. Natl. Acad. Sci. USA 95:8467-8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rong, L., K. Gendron, B. Strohl, R. Shenoy, R. J. Wool-Lewis, and P. Bates. 1998. Characterization of determinants for envelope binding and infection in Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 72:4552-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider, P., J. L. Bodmer, M. Thome, K. Hofmann, N. Holler, and J. Tschopp. 1997. Characterization of two receptors for TRAIL. FEBS Lett. 416:329-334. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan, J. P., S. A. Marsters, R. M. Pitti, A. Gurney, M. Skubatch, D. Baldwin, L. Ramakrishnan, C. L. Gray, K. Baker, W. I. Wood, A. D. Goddard, P. Godowski, and A. Ashkenazi. 1997. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277:818-821. [DOI] [PubMed] [Google Scholar]
- 45.Siegel, R. M., J. K. Frederiksen, D. A. Zacharias, F. K. Chan, M. Johnson, D. Lynch, R. Y. Tsien, and M. J. Lenardo. 2000. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science 288:2354-2357. [DOI] [PubMed] [Google Scholar]
- 46.Smith, E. J., J. Brojatsch, J. Naughton, and J. A. T. Young. 1998. The CAR1 gene encoding a cellular receptor specific for subgroup B and D avian leukosis viruses maps to the chicken tvb locus. J. Virol. 72:3501-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snitkovsky, S., T. M. J. Niederman, B. S. Carter, R. C. Mulligan, and J. A. T. Young. 2000. A TVA-single-chain antibody fusion protein mediates specific targeting of a subgroup A avian leukosis virus vector to cells expressing a tumor-specific form of epidermal growth factor receptor. J. Virol. 74:9540-9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snitkovsky, S., T. M. J. Niederman, R. C. Mulligan, and J. A. T. Young. 2001. Targeting avian leukosis virus subgroup A vectors by using a TVA-VEGF bridge protein. J. Virol. 75:1571-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snitkovsky, S., and J. A. Young. 1998. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. USA 95:7063-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walczak, H., M. A. Degli-Esposti, R. S. Johnson, P. J. Smolak, J. Y. Waugh, N. Boiani, M. S. Timour, M. J. Gerhart, K. A. Schooley, C. A. Smith, R. G. Goodwin, and C. T. Rauch. 1997. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 16:5386-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weller, S. K., A. E. Joy, and H. M. Temin. 1980. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J. Virol. 33:494-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weller, S. K., and H. M. Temin. 1981. Cell killing by avian leukosis viruses. J. Virol. 39:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wigler, M., S. Silverstein, L. S. Lee, A. Pellicer, Y. Cheng, and R. Axel. 1977. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell 11:223-232. [DOI] [PubMed] [Google Scholar]
- 54.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimoto, T., E. Yoshimoto, and D. Meruelo. 1993. Identification of amino acid residues critical for infection with ecotropic murine leukemia retrovirus. J. Virol. 67:1310-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young, J. A. T., P. Bates, and H. E. Varmus. 1993. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J. Virol. 67:1811-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zavorotinskaya, T., and L. M. Albritton. 1999. A hydrophobic patch in ecotropic murine leukemia virus envelope protein is the putative binding site for a critical tyrosine residue on the cellular receptor. J. Virol. 73:10164-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zingler, K., C. Bélanger, R. Peters, E. Agard, and J. A. T. Young. 1995. Identification and characterization of the viral interaction determinant of the subgroup A avian leukosis virus receptor. J. Virol. 69:4261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zingler, K., and J. A. T. Young. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]