Abstract
It has been generally believed that oncoretroviruses are dependent on mitosis for efficient nuclear entry of viral DNA. We previously identified a nuclear localization signal in the integrase protein of an oncoretrovirus, avian sarcoma virus (ASV), suggesting an active import mechanism for the integrase-DNA complex (G. Kukolj, R. A. Katz, and A. M. Skalka, Gene 223:157-163, 1998). Here, we have evaluated the requirement for mitosis in nuclear import and integration of ASV DNA. Using a modified ASV encoding a murine leukemia virus amphotropic env gene and a green fluorescent protein (GFP) reporter gene, DNA nuclear import was measured in cell cycle-arrested avian (DF-1) as well as human (HeLa) and mouse cells. The results showed efficient accumulation of nuclear forms of ASV DNA in γ-irradiation-arrested cells. Efficient transduction of a GFP reporter gene was also observed after infection of cells that were arrested with γ-irradiation, mitomycin C, nocodazole, or aphidicolin, confirming that nuclear import and integration of ASV DNA can occur in the absence of mitosis. By monitoring GFP expression in individual cells, we also obtained evidence for nuclear import of viral DNA during interphase in cycling cells. Lastly, we observed that ASV can transduce postmitotic mouse neurons. These results support an active nuclear import mechanism for the oncoretrovirus ASV and suggest that this mechanism can operate in both nondividing and dividing cells.
Early events in the retroviral replication cycle include reverse transcription of the viral RNA and integration of the resulting viral DNA genome into the host cell chromosomes (6, 12). These two steps are catalyzed by the viral enzymes reverse transcriptase (RT) and integrase (IN), which are carried into the cell within the virion capsid. In addition to the enzymatic steps, these early events require trafficking of the viral DNA, which is synthesized in the cytoplasm, to sites of integration in the host cell chromosomes in the nucleus. The precursor to the integrated viral DNA is a linear double-stranded DNA which is found in a subviral preintegration complex that contains IN, as well as other host and viral proteins. It is likely that all retroviruses utilize active mechanisms (as opposed to passive diffusion) for subcellular trafficking of viral DNA, and such movement may be facilitated by interactions between the preintegration complex and host cell factors or structures. As efficient and stable expression of viral DNA requires integration into the host chromosome, the transport of viral DNA to an integration site is essential to complete the early steps in infection. Integration of the viral DNA marks a transition to late steps that include synthesis of viral RNAs and proteins, followed by assembly and budding of progeny particles.
The nuclear membrane is a potential barrier for movement of retroviral DNA (in the preintegration complex) to the chromosomal integration sites. In dividing cells, which are progressing through the cell cycle (G1 → S → G2 → M), the nuclear membrane remains intact during interphase (G1 → S → G2) and disassembles during mitosis (M). During interphase, the movement of cellular components into the nucleus occurs through the nuclear pores. In postmitotic, nondividing cells, the nuclear membrane remains intact, and the nuclear pore is likely an obligatory pathway for nuclear entry of cellular and retroviral components (8). Retroviral transduction (reverse transcription and DNA integration) requires that the viral DNA enter the nucleus, either during mitosis, through the nuclear pore, or by some other active mechanism.
The host cell cycle requirements for support of retroviral infection can be studied using synchronized dividing cells or nondividing cells. Experimentally, nondividing cells include cycle-arrested cells (e.g., G2 arrest), quiescent cells that are temporarily withdrawn from the cell cycle (G0 cells; unstimulated or serum-starved cells) or terminally differentiated cells (G0 cells) that do not reenter the cell cycle. Early characterization of the prototypic retrovirus, Rous sarcoma virus (an avian sarcoma virus [ASV]; an alpharetrovirus), indicated that chicken embryo fibroblasts whose growth was arrested by serum starvation (G0 arrest) could not support efficient reverse transcription (14, 50). If cells were infected after release from G0 [(G0) → G1 → S → G2 → M], the available evidence suggested that reverse transcription and integration could take place during S phase (20, 50), implying active nuclear import of viral DNA prior to mitosis and cytokinesis. Mitosis appeared to be required for later, postintegration events necessary for production of progeny virions (20). Like ASV, the oncoretrovirus murine leukemia virus (MuLV; a gammaretrovirus) requires cell cycling to establish a productive infection (17, 36). Furthermore, detailed studies by Roe et al. (44) indicated that MuLV DNA enters the nucleus primarily during mitosis, suggesting that nuclear membrane breakdown facilitated this process.
The discovery that human immunodeficiency virus type 1 (HIV-1) could infect nondividing cells (29, 51) suggested an active import mechanism that was independent of mitosis; active nuclear pore-mediated import of HIV-1 DNA in nondividing cells was subsequently demonstrated (4, 5). Several HIV-1 determinants have been implicated in nuclear import in nondividing cells, but the field has been controversial (8, 13). The mechanism of nuclear import of HIV-1 DNA in dividing cells is not well understood, but some evidence suggests that HIV-1 also exploits nuclear membrane breakdown during M phase (4). Studies of the HIV-1 nuclear import mechanism have used cycle-arrested, quiescent, and terminally differentiated cells. As with ASV, early events of HIV-1 infection, including reverse transcription, can be restricted in quiescent (G0) cells (11). However, HIV-1 can transduce certain nondividing, differentiated cells such as neurons and macrophages (37, 38, 51), presumably also arrested in G0.
Direct comparisons have demonstrated that HIV-1-based vectors can transduce nondividing cells much more efficiently than MuLV vectors (2, 4, 29, 30, 38, 43, 49). This difference has been attributed largely to a lack of nuclear import function for MuLV. Extrapolating from results with MuLV (30, 44), it has been widely assumed that all oncoretroviruses require mitosis for efficient nuclear entry of viral DNA. The early studies implying active nuclear import for ASV (20, 50) have been either reinterpreted (44) or ignored. Our motivation for challenging this assumption comes from the observation that ASV IN, a component of the preintegration complex, localizes to the nucleus when expressed independently (25). Furthermore, a noncanonical nuclear localization signal (NLS) was mapped to positions 207 to 235 of IN and residues in this NLS were found to be critical for IN nuclear import (26). These findings suggested that ASV could use an active, nuclear pore-mediated import mechanism for DNA entry into the nucleus. To obtain a better understanding of the cell cycle dependence of retroviral replication, we have examined the ability of ASV to infect nondividing cells using a variety of conditions and assay systems. Our results show that ASV DNA can be imported efficiently into the nucleus during interphase in cells arrested by a variety of methods. In addition, we provide evidence that this nuclear import mechanism can be used in dividing cells during interphase, as well as in nondividing differentiated cells. These results extend our understanding of the virus-host interactions and have implications for the design of retroviral vectors and antiretroviral therapies.
MATERIALS AND METHODS
Cells.
The DF-1 chicken embryo fibroblast line was obtained from D. Foster and maintained as described (45). The FT210 cell line was obtained from E. M. Bradbury (with permission from F. Hanaoka) and was propagated as described (48).
Vector construction and viruses.
The starting ASV vectors were derived from the RCAS series, in which the ASV (Rous sarcoma virus) src gene can be replaced with reporter genes or selectable markers. The RCASBP M2C (4070A) Puro vector was obtained from S. Hughes (1). This RCAS vector encodes an MuLV amphotropic env gene which allows entry into mammalian cells. The RCASBP M2C (4070A) vector backbone also contains an adaptive mutation in the amphotropic env gene that allows more efficient replication in chicken cells. This vector was digested with ClaI to release the puromycin gene and a cassette containing the cytomegalovirus (CMV) immediate-early promoter driving the gene for enhanced green fluorescent protein (EGFP) was inserted. The CMV-EGFP segment was amplified from the pEGFP-C2 expression clone (Clontech) by PCR. The downstream primer was positioned such that the pEGFP-C2 simian virus 40 poly(A) signal was not included in the amplified cassette. ClaI restriction sites were included in the PCR primers to facilitate cloning of the cassette into the ClaI-cleaved RCAS vector. The orientation of the cassette was sense, such that the poly(A) site in the downstream viral long terminal repeat (LTR) would be used for 3′-RNA processing (see Fig. 2). The final construct (pASVA-CMVEGFP) was used to transfect DF-1 chicken cells using the DEAE-dextran method. Over a 2-week period, the cells were passaged and the virus spread through the culture as indicated by efficient transduction of the EGFP gene. Once the culture was uniformly infected, the cultures were maintained as producer cells without serial passage of the virus (to minimize loss of the EGFP cassette during reverse transcription). The vector could efficiently transduce the EGFP gene to HeLa and other mammalian cells, as expected. Typically, titers of more than 106 transducing units per ml were obtained when the virus was assayed on HeLa cells. Only a single round of transduction by the ASV vector can occur in these mammalian cells, as several post-DNA integration steps are restricted compared to fully permissive avian cells (1).
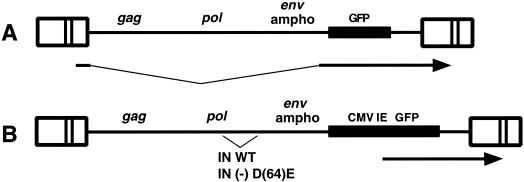
FIG. 2.
Maps of viruses ASVA-GFP (A) and ASVA-CMVEGFP (B). Arrows indicate GFP expression from spliced RNA or CMV-EGFP cassette. LTRs are indicated by boxes. WT, wild type.
A second construct was prepared from the RCASBP M2C (4070A) in which the GFP is translated from a spliced RNA expressed from the LTR. The GFP gene was obtained from an RCAS(A)-GFP plasmid provided by C. Cepko. The GFP coding sequence was removed from RCAS(A)-GFP by cleavage with ClaI and inserted into the ClaI site in RCASBP M2C (4070A). The resulting DNA clone (pASVA-GFP) was used to transfect DF-1 cells as described above. The resulting virus behaved similarly to the virus generated from pASVA-CMVEGFP, except that the transducing titer was ca. 5-fold reduced as measured on HeLa cells.
The MuLV amphotropic virus was obtained as plasmid DNA (pAMS) from the American Type Culture Collection (ATCC no. 45167). NIH 3T3 cells were transfected with pAMS, and virus production was monitored by Western blotting using an anti-MuLV antibody kindly provided by M. Roth.
Infection and analysis of GFP expression.
For experiments with ASVA-CMVEGFP or ASVA-GFP, undiluted supernatants from producer DF-1 cultures were used. Prior to infection, the virus-containing medium was passed through a 0.45-μm-pore-size filter to remove contaminating DF-1 producer cells. Target cells were infected with the filtered virus for 2 h in the presence of DEAE-dextran (10 μg/ml), and GFP expression was typically observed 24 to 72 h postinfection. Microscopy was carried out using a Nikon Eclipse TE800 inverted microscope fitted with a charge-coupled device camera or a Nikon COOLPIX 950 digital camera. Transduction, as measured by GFP expression, was quantitated using a Becton Dickinson FACScan flow analyzer. Infected cells were analyzed on a green-versus-orange fluorescence plot with 14.5% orange minus green compensation and 0.8% green minus orange compensation.
Cell cycle arrest and synchronization.
Cells were arrested by exposure to 40 Gy of γ-irradiation using cesium-137 irradiators. The Shepherd model 280 was used for DF-1 cells, which were suspended after trypsinization. A cesium-137 panoramic irradiator, Shepherd model 81-14R, was used for HeLa cell monolayers. Cell cycle status was determined by DNA content using a Becton Dickinson FACScan flow analyzer. DF-1 and HeLa cells were arrested by treatment with mitomycin C at a concentration of 10 μg/ml for 30 min (34). G1/S arrest of DF-1 cells was established by treatment with aphidicolin (2 to 5 μg/ml) for 24 h, and M-phase arrest of DF-1 cells was established by treatment with nocodazole (25 μg/ml). For synchronization of HeLa cells, cultures were treated with nocodazole (16 ng/ml) for 4 h and mitotic cells were removed by tapping the plate. For G1/S arrest of HeLa and DF-1 cells, 2 and 0.5 mM hydroxyurea was used, respectively.
PCR analyses.
Circular retroviral DNA, a marker for nuclear import, was detected by PCR using DNA primer pairs that specifically amplify the LTR junctions formed when linear viral is circularized (5). Cells were infected as described above and Hirt supernatant DNA was prepared at 16 to 24 h postinfection by standard methods.
PCR primers for ASV were 5′-TTATTGTATCGAGCTAGGCAC-3′ and 5′-CATCGAGCACCTGCATGAAGC-3′.
PCR primers for MuLV were 5′-GTGGTCTGCTGTTCCTTGG-3′ and 5′-GGGGCACCCTGGAAACATCT-3′. The 5′ end of one primer in each pair was labeled with 32P. PCRs were carried out for 25 to 30 cycles and were determined to be in the quantitative range. Products (ca. 300 bp) were fractionated on 7% urea-acrylamide gels.
Hippocampal neuron explants.
Explants were prepared from C57BL/6 mouse embryos (embryonic day 14.5 [E14.5]) as described previously (27).
RESULTS
ASV DNA can enter the nuclei of arrested cells.
HIV-1 DNA has been shown to enter the nuclei of cells whose division has been blocked within the cell cycle by a variety of treatments (3-5, 29-31). Nuclear import of retroviral DNA is routinely monitored by the appearance of closed circular molecules in which sequences at the linear ends (LTRs) are joined precisely (LTR junctions) (Fig. 1A). Although such circular molecules are dead-end products and not substrates for integration, they provide a convenient measure for nuclear entry, as their appearance is likely to be dependent on the action of nuclear DNA ligases. To investigate nuclear entry for ASV DNA, we used derivatives of the replication-competent ASV vector that encodes an MuLV amphotropic env gene (RCASBP-M) (1, 9). This vector has an extended host range, allowing analyses to be carried out in both avian and mammalian cells. In mammalian cells, ASV is competent for early steps of infection including integration, while virus production is blocked (1, 6). Therefore, in experiments using HeLa cells, we could analyze results of single transduction events without further virus spread.
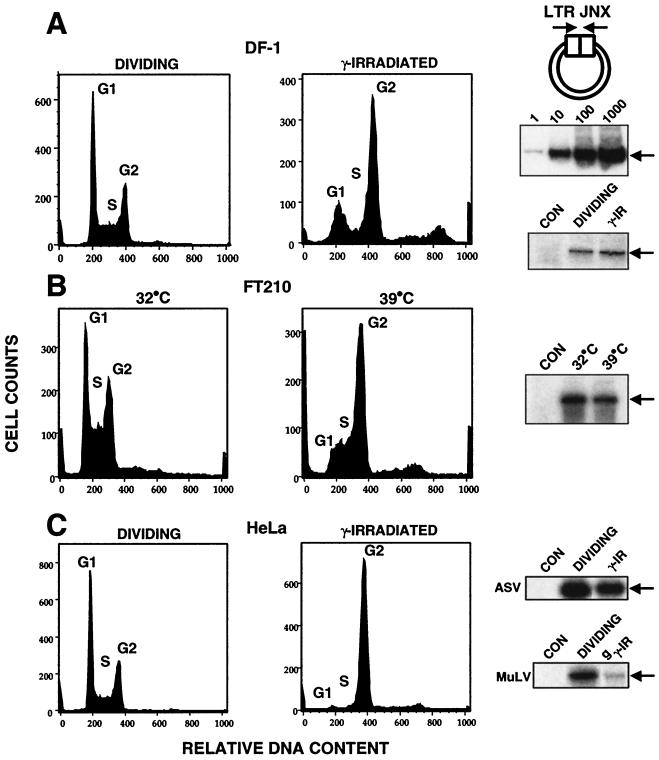
FIG. 1.
Nuclear import of retroviral DNA in arrested cells. Viral DNA LTR junctions signify nuclear entry of viral DNA. In each panel, the cell cycle profiles are shown along with gel analyses of the PCR product corresponding to LTR junctions (LTR JNX [arrows]). FACS analyses for DNA content indicate cell cycle status. Cell cycle profiles shown on the left represent dividing cells, and those on the right are for arrested cells. Dividing and arrested cells were exposed to the ASV amphotropic virus for 2 h, and DNA was isolated 16 to 24 h postinfection for PCR analyses. PCR analysis of mock-infected cells (CON) is shown. Cell cycle profiles were obtained from separate experiments using identical conditions. (A) DF-1 chicken embryo fibroblast line. At top right, a diagram of the PCR strategy is shown; the middle panel presents a standard curve for PCR showing a linear response over 3 to 4 logs. (B) FT210 cell line. (C) HeLa cell line. See Materials and Methods for details.
To provide a basis for comparison with natural host cells, we first assayed ASV DNA nuclear import in the chicken embryo fibroblast DF-1 cell line. After exposure to γ-irradiation, cell cycle arrest was evident and was monitored by DNA content by fluorescence-activated cell sorting (FACS) (Fig. 1A). The results verified that cells were arrested in G1 or G2. The DF-1 cells were infected 24 h postirradiation with a replication-competent ASV vector, and viral DNA was analyzed 16 to 24 h postinfection. The appearance of ASV LTR junctions indicated that ASV DNA was imported into the nuclei of the arrested cells with an efficiency similar to that for dividing cells (Fig. 1A).
We also utilized a temperature-sensitive mouse cell line (FT210) that can be arrested in G2 by shifting from 32°C (permissive temperature) to 39°C (nonpermissive temperature). FT210 cells were maintained at the nonpermissive temperature for 20 h, and cell cycle arrest was confirmed by FACS analysis (Fig. 1B). These cells were infected with an ASV amphotropic vector, RCASBP-M, and the assay for LTR junctions was performed on cells harvested 16 h postinfection. As shown in Fig. 1B, LTR junctions were readily detected in the cultures that were shifted to 39°C.
It has been reported that HIV-1 DNA is able to enter the nucleus of HeLa cells that have been arrested in G2 by exposure to γ-irradiation (4, 10, 29, 37). HeLa cells were arrested in G2 with γ-irradiation and infected with the ASV vector, using conditions identical to those described in the HIV-1 studies (29). A parallel arrested culture was also infected with MuLV also encoding an amphotropic envelope gene. FACS analysis confirmed that the HeLa cells were uniformly arrested in G2 at the time of infection, as expected (Fig. 1C). The results showed efficient formation of ASV LTR junctions in the G2-arrested HeLa cells, compared to the amount observed in dividing HeLa cells (Fig. 1C). MuLV LTR junctions were also detectable in the arrested HeLa cells, but the amount was significantly reduced compared to that in dividing cells. From these results we conclude that ASV DNA is imported efficiently into the nuclei of arrested cells.
Construction of ASV vectors expressing a GFP reporter gene and experimental design.
To evaluate ASV DNA import further, we designed ASV amphotropic vectors that encode a GFP reporter protein. As nuclear functions are required for transcription and processing of vector RNA encoding GFP, expression of GFP is an unequivocal indicator of nuclear import of the retroviral DNA transcriptional template. Two vectors were designed (Fig. 2), both based on the ASV amphotropic virus described above (1). In one vector, the EGFP open reading frame was inserted into the src position such that it is expressed from a spliced RNA transcribed from the viral LTR. In the second vector, GFP expression is driven by the CMV immediate-early promoter. Chicken DF-1 cells were transfected with the two DNA constructs, and the progeny virus spread through the culture, as monitored by expression of GFP. The vector that included the CMV-driven GFP displayed a 5- to 10-fold higher titer on HeLa cells than the vector that expressed GFP from the viral LTR (data not shown). This difference likely reflects the more efficient expression of the GFP reporter gene from the CMV promoter. To examine possible expression from unintegrated DNA, we engineered an IN-inactivating [IN(−)] mutation (D64E) (9) into the CMV-EGFP version of the vector (Fig. 2B). Infection with the IN(−) vector did not produce detectable GFP expression in HeLa cells, as determined by microscopy or by FACS analysis, indicating that integration was required for efficient GFP expression (data not shown). Therefore, in the experiments described below, we interpret GFP expression as indicative of completion of all early events: reverse transcription, nuclear entry, and integration of viral DNA (i.e., transduction of the GFP reporter). We note that reporter expression from the CMV-EGFP construct does not require that the ASV LTRs be transcriptionally active. In this study we have focused on CMV-driven GFP expression, and it is possible that in natural infections, the LTR-driven transcription might be influenced by cell cycle or the host integration site.
ASV transduces cells arrested with nocodazole.
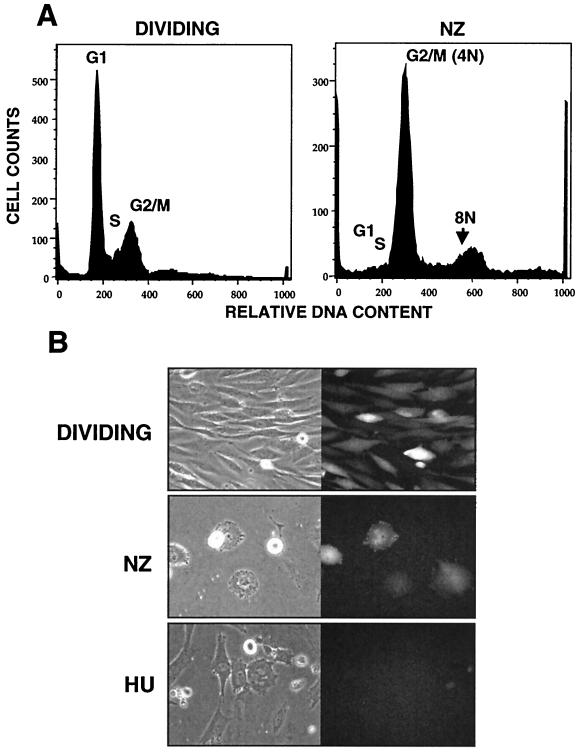
The DNA import that we observed in arrested cells (Fig. 1) is consistent with the early studies by Humphries and coworkers (20), which indicated that ASV DNA could be integrated in synchronized chicken cells prior to mitosis. As a further test of this interpretation, we performed a similar infection with synchronized cells. Chicken DF-1 cells were arrested in G1/S by treatment for 24 h with hydroxyurea (DNA content profile not shown). The cells were then released by removal of hydroxyurea and subsequently infected with the ASV-GFP vector in the presence of nocodazole to prevent passage through M phase. Arrest at M phase was confirmed by cell sorting for DNA content (Fig. 3). Thirty-six hours after infection, approximately 50% of the nocodazole-treated cells expressed GFP, indicating that efficient integration and expression could occur in the absence of cytokinesis (Fig. 3). Although these cells were arrested efficiently, the morphology of those that remained attached to the dish was distinct from the characteristically round, highly refractile mitotic cells. In certain genetic backgrounds, prolonged exposure to mitotic inhibitors can result in chromosome decondensation and resumption of DNA synthesis in the absence of cytokinesis (7). Consistent with this possibility, we observed a peak corresponding to 8N DNA content or more after only 24 h of nocodazole exposure (Fig. 3). We also found that cells exposed to nocodazole continuously for 24 h preinfection through 36 h postinfection were readily transduced by the ASV-GFP vector (data not shown). As nocodazole affects microtubule dynamics and does not prevent nuclear membrane breakdown, this experiment cannot provide evidence for pore-mediated nuclear import of ASV DNA. However, the results do indicate that microtubule dynamics or cytokinesis is not required for efficient transduction by ASV.
FIG. 3.
Infection of nocodazole- and hydroxyurea-arrested cells with ASV. (A) Cell cycle profiles of dividing and nocodazole-arrested DF-1 cells obtained after 24 h of treatment (from a separate experiment). (B) DF-1 cells were synchronized by 24 h of treatment with hydroxyurea (HU) and infected with ASVA-GFP in the presence of nocodazole. Cells were maintained in nocodazole (NZ) for an additional 36 h, at which time micrographs were taken. DF-1 cells were arrested with HU for 24 h, infected for 2 h, and maintained in HU for 48 h. Results with control dividing cultures are also shown. Shown are phase-contrast (left) and fluorescence (right) micrographs of dividing and arrested cells 48 h postinfection.
Hydroxyurea inhibits transduction by ASV.
Hydroxyurea inhibits ribonucleotide reductase, resulting in a depletion of deoxyribonucleotide precursors and cell cycle arrest at G1/S. To test for a requirement for S phase we arrested chicken DF-1 cells at G1/S with hydroxyurea. We observed that the hydroxyurea-arrested DF-1 cells were highly resistant to transduction with the ASV-GFP vector (Fig. 3), as expected (47), although rare GFP-positive cells could be detected. In separate experiments, we found that reverse transcription was highly compromised in hydroxyurea-treated cells, as measured 16 to 24 h postinfection by PCR (data not shown). The reduction in deoxyribonucleotide pool size by hydroxyurea treatment is thought to adversely affect retroviral reverse transcription (15, 16, 33, 35). Thus, the severe reduction in the number of GFP-positive cells observed after treatment with hydroxyurea is likely due, in large part, to inhibition of reverse transcription. If hydroxyurea was removed 5 h postinfection, the number of GFP-expressing cells was similar to that observed with dividing cells. However, efficient transduction was not observed if cells were released after longer hydroxyurea treatment (data not shown). These observations suggest that the incoming ASV subviral complex is labile if the cellular environment does not support efficient reverse transcription.
Due to the severe effects of hydroxyurea on reverse transcription, nuclear import could not be assayed in cells arrested in G1/S by this method. A small amount of the LTR junction species could be detected after infection of hydroxyurea-arrested cells (data not shown); however, it cannot be determined if these LTR junctions were formed in rare arrested cells in which reverse transcription was completed or in rare cells that escaped cell cycle arrest. In any case, these results indicate that efficient transduction of the GFP reporter by the ASV vector requires reverse transcription and cannot be ascribed to artifactual pseudotransduction, mediated by incoming viral RNA. These results also show that ASV reverse transcription or some other step is likely sensitive to deoxyribonucleotide pool levels and that deficiencies in the concentration of these substrates can restrict transduction.
Aphidicolin-arrested cells are transduced by ASV.
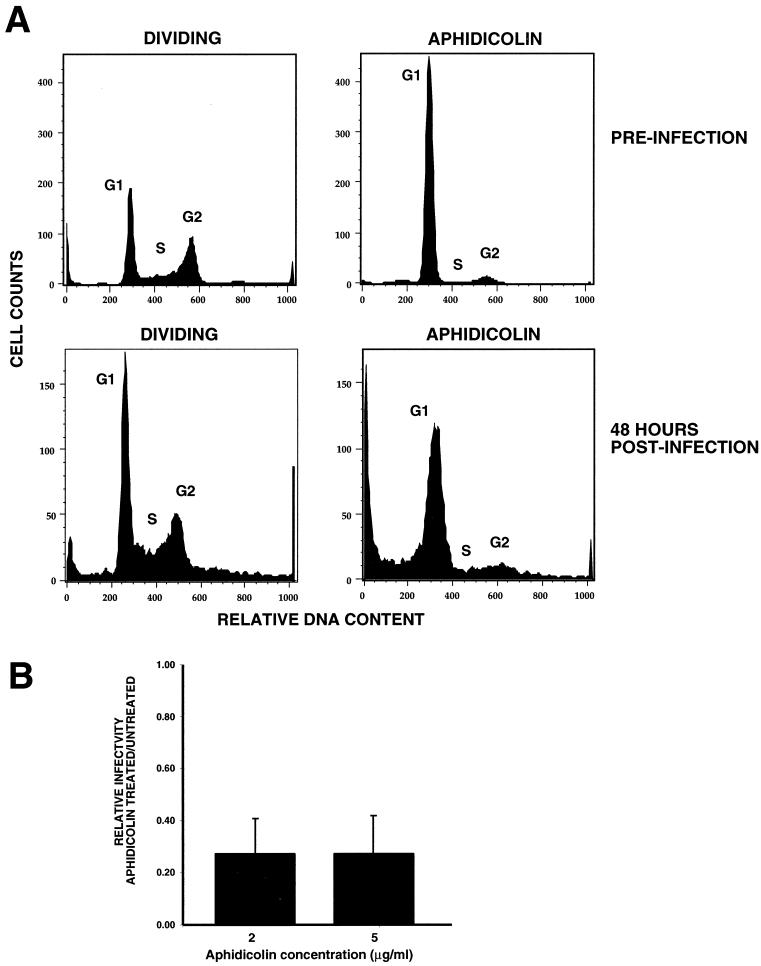
Because cells arrested with hydroxyurea did not support efficient reverse transcription, we used a second inhibitor, aphidicolin, which also causes arrest at G1/S. DF-1 cells were treated with aphidicolin, and the expected cell cycle arrest was observed (Fig. 4A). Forty-eight hours postinfection, GFP expression was observed with an efficiency of about 30% of that of dividing cells (Fig. 4B). This relative efficiency was observed over a wide multiplicity of infection range among the triplicate experiments. This dose-response relationship indicates that the experimental design allows a suitable quantitation of the relative infectibility of these cycling and arrested cells. These results further support an active DNA import mechanism during interphase.
FIG. 4.
Infection of aphidicolin-arrested cells with ASV. (A) Cell cycle profiles of dividing and aphidicolin (5 μg/ml)-treated DF-1 cells. In the top panels, DF-1 cells were pretreated with aphidicolin for 24 h prior to infection with ASVA-CMVEGFP (preinfection profile). The bottom panels show the cell cycle profile of aphidicolin-treated cells 48 h postinfection (72 h total exposure). (B) Transduction of aphidicolin-arrested cells relative to dividing cells 48 h postinfection. GFP-expressing cells were measured by FACS analysis. Results at each concentration of aphidicolin were averaged from three separate experiments, and the standard deviation (error bar) is shown.
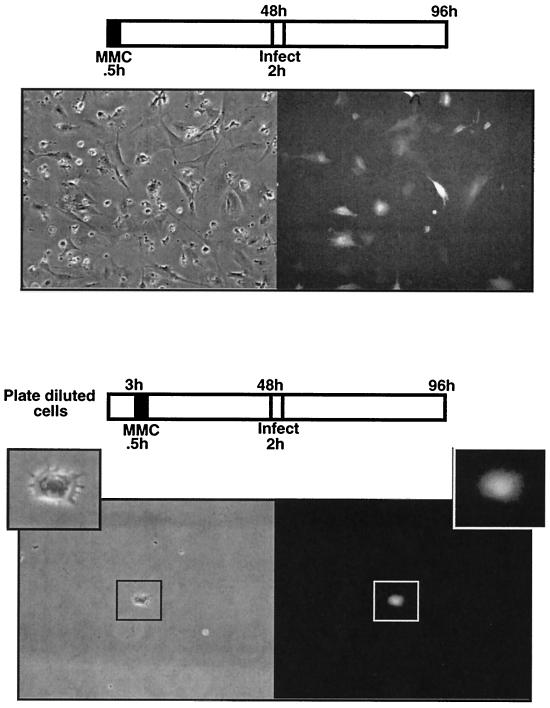
Mitomycin C-arrested cells are transduced by ASV. Mitomycin C is a DNA-damaging agent routinely used to prepare cell feeder layers (34). Cells are treated briefly with mitomycin C and by 48 h, cell cycle arrest is uniform and permanent (typically in G2). DF-1 cells were treated with mitomycin C for 0.5 h, cultured for 48 h, and then infected with the ASVA-CMVEGFP vector (Fig. 2). As illustrated in Fig. 5 (top panels) GFP expression was observed in more than 50% of the arrested cells at 36 to 48 h postinfection. Similar results were obtained with mitomycin C-arrested HeLa cells (data not shown).
FIG. 5.
Infection of mitomycin C-arrested DF-1 cells with ASV. In the top panels, DF-1 cells were treated with mitomycin C for 0.5 h. After 48 h, cells were infected with ASVA-CMVEGFP and examined for GFP expression 48 h postinfection. Phase-contrast (left) and fluorescence (right) images (GFP) are shown. The bottom panels are the same as the top panels, except that DF-1 cells were monodispersed prior to mitomycin C treatment such that infection of individual cells could be observed. Shown is an arrested premitotic cell expressing GFP.
To test the unlikely possibility that infected cells arose via escape from mitomycin C arrest under the conditions we used, we followed infection of individual cells. DF-1 cells were plated under dilute conditions, and 3 h postplating, the cells were treated with mitomycin C for 0.5 h and then cultured for 48 h prior to infection. Some cells underwent a final division within 48 h of treatment (as expected), prior to infection, but did not divide subsequently, as we observed a maximum of two cells per colony. As shown in Fig. 5 (bottom panels) single GFP-expressing cells were observed that had not divided prior to or subsequent to infection. These results confirm that ASV transduction can occur independent of mitosis.
We also measured virus production from mitomycin C-arrested DF-1 cells. A culture dish of transduced (GFP-expressing), arrested DF-1 cells was assayed for virus production by transferring undiluted supernatants to cycling HeLa cell cultures and scoring for transduction of the GFP reporter. As we could not detect any infected HeLa target cells, we estimate that virus production from mitomycin C-treated DF-1 cells is reduced by at least 5 logs compared to dividing DF-1 cells (data not shown). Taken together, our results are consistent with earlier observations that efficient ASV integration is mitosis independent but virus production is mitosis dependent (20). We note that in the transduced, arrested cells, the GFP reporter is expressed from the CMV immediate-early promoter, while virus production requires activity of the LTR. It is possible that viral gene expression from the LTR is cell cycle restricted in these arrested cells or that some other postintegration step is limited. As mitomycin C arrest is irreversible, further studies with reversible inhibitors will be required to rigorously test the requirement for mitosis for virus production.
HeLa cells arrested in G2 are transduced efficiently by ASV.
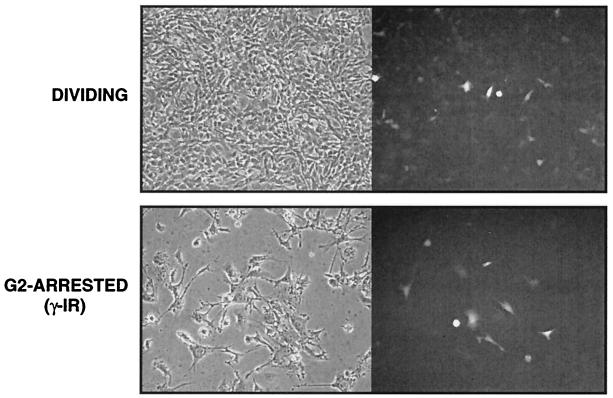
The data summarized in Fig. 1 indicate that G2-arrested HeLa cells could support reverse transcription and active ASV DNA nuclear import. However, because the viral LTR junction-containing DNA molecules which are indicators of nuclear entry are dead-end products with respect to integration, it was important to verify that biologically active DNA is also imported into the nucleus. Accordingly, we infected G2-arrested (γ-irradiated) HeLa cells with the ASV vector (RCASA-CMVEGFP) under arrest conditions identical to those previously described for HIV-1 (29) (as in Fig. 1). As shown in Fig. 6, we observed efficient expression of GFP (>70%) in cycling HeLa cells, indicating that the amphotropic pseudotyped ASV vector can integrate efficiently and transduce cycling human cells, as expected. We also observed highly efficient transduction of the G2-arrested HeLa cells (ca. 50% of the efficiency observed with cycling cells). These results indicate that G2-arrested HeLa cells can support all early steps in ASV infection, including active nuclear import and integration of viral DNA.
FIG. 6.
Infection of γ-irradiated (γ-IR) HeLa cells with ASV. HeLa cells were arrested in G2 as described in Fig. 1 and were infected with ASVA-CMVEGFP 24 h later. Shown are phase-contrast (left) and fluorescence (right) micrographs of dividing and arrested cells 48 h postinfection.
Evidence for active nuclear import of ASV DNA during interphase in dividing cells.
The studies described above show that ASV DNA is efficiently imported into nuclei of cycle-arrested cells compared to dividing cells and that cycle-arrested cells could be transduced. To determine if active nuclear import also occurs in dividing cells, we prepared synchronized cells that could be monitored for GFP expression in relation to M phase. Monodispersed, M-phase HeLa cells were prepared by mitotic shake-off from nocodazole-treated cultures. The cells were confirmed to be ca. 90% 4N DNA content by FACS analysis (data not shown). These cells were plated under dilute conditions and were allowed to attach to the culture dish. By 3 h postplating, 70 to 90% of the single mitotic cells had entered G1, as indicated by the appearance of cell doublets (two adjacent nucleated cells). A subset of these cell doublets (ca. 100) was marked for later identification. The cultures were then infected with the ASV vector and observed for GFP expression 20 to 48 h postinfection. As shown in Fig. 7, GFP expression could be observed in cell doublets prior to further division. In such cells, import of viral DNA into the nucleus must have taken place during interphase, i.e., prior to the next mitosis. Subsequent analyses of GFP expression during colony outgrowth confirmed that integration had occurred during interphase (R. A. Katz et al., unpublished data). The results presented here suggest that all early steps in ASV replication cycle can be completed during interphase in dividing cells. We note that although the design of experiments described in Fig. 7 provides qualitative evidence for transduction during interphase, the results are not readily quantifiable. However, additional experimental results indicate that the ASV DNA nuclear import and integration can occur efficiently during interphase in cycling cells (Katz et al., unpublished data).
FIG. 7.
Evidence for ASV DNA nuclear import during interphase in dividing cells. HeLa cells were synchronized by mitotic shake-off. Dilute mitotic cells completed cytokinesis, producing two-cell colonies. Three hours after plating, cultures were infected with ASVA-CMVEGFP for 2 h. Cells were observed for GFP expression over the next 24 to 36 h. Phase-contrast (left) and fluorescence (right) images (GFP) are shown. Two fields are shown. The two-cell colony in the lower panel was marked prior to infection.
ASV transduction of postmitotic neurons.
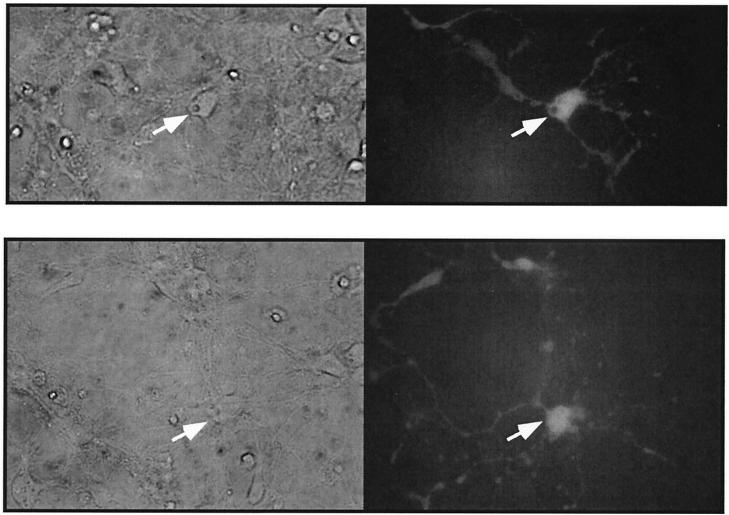
The results described above establish that ASV DNA is imported into nuclei of both cycle-arrested and dividing cells during interphase. We next asked if ASV could transduce naturally arrested, terminally differentiated cells in culture. For these experiments, we used mouse embryonic hippocampal neuron microexplants (27) that are isolated between E14.5 and E16.5 during the transition from neuroepithelial progenitor cells to differentiated neurons. After establishing the explant, the neuroepithelial cells immediately undergo a single division and then become highly differentiated within 4 days. Cultures were infected with the ASV vector on day 2, 5, or 14 postexplantation and then monitored for GFP expression 48 to 72 h postinfection. GFP expression was observed in ca. 5% of the explanted cells infected on day 2 postexplantation, with only a slight reduction in those cultures infected on days 5 and 14. We consider this level of transduction to be efficient, as we typically observed ca. 20% infection of dividing NIH 3T3 cells under similar conditions (data not shown). A large proportion of the GFP-expressing cells had distinct neuronal morphology (Fig. 8). As there is no detectable division of differentiated neurons in explants cultured for 5 and 14 days, we interpret our results to mean that all early steps of ASV replication, including viral DNA nuclear entry and integration, can occur in these differentiated neurons. Other results indicate that detectable transduction of neurons with an MuLV-GFP vector is observed only if explants are infected on day 2 postexplantation, at which time division of progenitor cells occurs (data not shown).
FIG. 8.
Infection of mouse neurons with ASV. Hippocampal neuron explants were prepared from mouse embryos and infected with ASVA-CMVEGFP on day 5 postexplantation. See Results and Materials and Methods for details. Phase-contrast (left) and fluorescence (right) images (GFP) are shown. Arrows identify the cell body. Two fields are shown.
DISCUSSION
It has been observed widely that productive infection and/or transduction by oncoretroviruses (e.g., MuLV and ASV) requires host cell division. Both S phase (20) and mitosis (44) have been implicated as being critical for propagation of these viruses. It is proposed that late G1 or S phase provides a supportive environment for efficient reverse transcription for ASV (20) and MuLV (40), as well as HIV-1 (22-24), while nuclear membrane breakdown during mitosis allows access to host chromosomes for MuLV DNA integration (44). Early studies suggested that reverse transcription and integration of ASV could occur during S phase (20, 50) and that passage through mitosis was required to activate the integrated viral DNA for expression and particle production (20). In assessing the role of the cell cycle in retroviral replication, there is an important distinction between productive infection and transduction. For the analysis of retroviral vectors, which are normally replication deficient, only the efficiency of transduction is usually considered. Our results indicate that mitosis is not essential for transduction by ASV; however, other aspects of the cell cycle may be required for productive infection.
In the present study, two methods were used to assess the requirement for mitosis for efficient nuclear entry and integration of ASV DNA. First, we show that nuclear forms of ASV DNA (containing LTR junctions) can be detected in both avian and mammalian cells that have been arrested by different methods to prevent mitosis. The amounts of these nuclear forms in arrested cells are comparable to those in dividing cells (Fig. 1). The relatively high levels of DNA import, compared to cycling cells, argues against passive import of the viral DNA into the nucleus. Similarly, it is unlikely that a subpopulation of dividing cells could account for these results; this is especially true for γ-irradiated HeLa cells in which G2 arrest is typically uniform (Fig. 1C). These results suggest that ASV uses an active nuclear import mechanism, likely via the nuclear pore. We were also able to detect a lesser amount of MuLV DNA in the nuclei of γ-irradiated HeLa cells, suggesting that it may also be imported actively, albeit at a lower efficiency than ASV. The second method that we used to monitor nuclear import of ASV DNA was through transduction of a GFP reporter. Efficient GFP expression was detected in cells arrested with γ-irradiation, nocodazole, mitomycin C, or aphidicolin (Fig. 3 through 6). We also monitored GFP reporter gene expression in individual arrested cells to rule out the possibility that ASV-mediated transduction was the result of leaky cell division (Fig. 5). As GFP expression was not observed after infection with an IN-deficient vector, we conclude that reporter expression requires integration in this system. Long-term expression (ca. 2 weeks) of GFP in mitomycin C-arrested cells also indicated that integration had likely occurred in arrested cells (data not shown).
Although mitomycin C-arrested chicken DF-1 cells could be transduced by ASV (as indicated by expression of the CMV-driven GFP reporter), these cells were unable to support detectable release of infectious particles. The mechanism of this restriction remains to be investigated but is consistent with the earlier report indicating that passage through mitosis was required for productive infection (20).
HIV-1 can transduce (and productively infect) certain nondividing cells. HIV-1 encodes multiple determinants that mediate active nuclear import of the viral DNA (8, 13), and it is believed that this allows HIV-1 to bypass the requirement for mitosis in nondividing cells. However, cell-cycle-specific components (2, 22-24, 39, 46, 52, 53) likely play an important role in the efficiency of HIV reverse transcription (and possibly integration). Notably, HIV-1 nuclear import determinants appear to be necessary but not sufficient for infection of all nondividing cells (11). For example, HIV-1 reverse transcription is compromised in quiescent (G0) T cells, likely due in part to the limitations in deoxyribonucleotide precursor pools and the transition to late G1 is required for completion of reverse transcription (24). Increasing the efficiency of reverse transcription in quiescent cells by boosting deoxyribonucleotide pools appears to be insufficient for transduction, indicating that other cell-cycle-specific components are limiting (23). HIV-1 is able to transduce terminally differentiated (presumably G0) cells such as neurons and macrophages; however, deoxyribonucleotide pools (2) or cell activation (22), respectively, can influence the efficiency of transduction. In contrast to limitations in quiescent cells, HIV-1 can transduce efficiently cycle-arrested cells (e.g., G2-arrested HeLa cells) (4, 10, 29, 37). We note a similarity in that ASV reverse transcription was initially shown to be blocked in quiescent cells (20), while here we demonstrate efficient transduction of cycle-arrested cells. It is possible that for both ASV and HIV-1, the ability to infect terminally differentiated (G0) cells may be limited by threshold levels of cellular components.
Our results indicate that ASV DNA import and integration can occur during interphase and are not dependent on mitosis. To prevent mitosis, cell cycle arrest was induced by γ-irradiation, mitomycin C, nocodazole, or aphidicolin. For successful transduction in cycle-arrested cells, the cell cycle phase in which the arrest occurs must be able to support early events (reverse transcription, DNA import, and integration). As mentioned, cellular components enriched during S phase (e.g., deoxyribonucleotide precursors) are important to support efficient retroviral reverse transcription (15, 16, 22, 23, 33, 35). Hydroxyurea treatment prevents entry into S phase by depleting deoxyribonucleotide precursor pools via inhibition of ribonucleotide reductase (33). We observed that ASV reverse transcription and transduction (Fig. 3) were highly compromised in hydroxyurea-arrested cells, as expected. This observation is therefore consistent with a dependency on S phase.
Aphidicolin also blocks entry into S phase by inhibiting DNA polymerase α. However, when asynchronous cells are treated, cells that are in S phase at the time of aphidicolin addition are arrested within S phase (21). In such cells, deoxyribonucleotide precursor levels are apparently preserved (42), and thus cells in S phase at the time of arrest may be able to support reverse transcription. Therefore, the ability of ASV to transduce aphidicolin-arrested cells (Fig. 4) is not at odds with a dependency on S phase. However, the efficiency of transduction of aphidicolin-arrested cells was reduced compared to that of cycling cells (Fig. 4). Early studies (19) indicated that aphidicolin inhibited circularization of ASV DNA as well as DNA integration, independently of cell cycle arrest. Recent studies have shown that the nonhomologous-end-joining pathway may be involved in the final repair of the retroviral integration intermediate (9), as well as circularization of unintegrated linear viral DNA (32). Aphidicolin may inhibit the nonhomologous-end-joining pathway (41), which is required for efficient retroviral transduction. Thus, it is possible that aphidicolin can have direct effects on transduction efficiency by inhibiting DNA repair at the integration site, although this remains to be investigated.
Cells arrested in mitosis with the microtubule inhibitor nocodazole could also support efficient transduction by ASV. The ability to transduce nocodazole-arrested cells also does not rule out a role for S phase, as DNA synthesis can reinitiate without cytokinesis after long-term exposure to microtubule inhibitors (Fig. 3) (7). We also observed that G2-arrested HeLa cells could be transduced efficiently by ASV, indicating that this cell cycle phase supported all early steps in viral replication, as is the case for HIV-1. It is possible that the required S-phase components (e.g., deoxyribonucleotide precursors and factors) remain in sufficient amounts to support transduction of G2-arrested cells. Taken together, our results appear to rule out a requirement for mitosis and support the idea that S phase is important for early events in ASV DNA replication.
The requirement for mitosis was also analyzed by monitoring ASV transduction (via GFP expression) in individual synchronized cells in real time. Synchronized HeLa cells infected during G1 were found to express GFP prior to mitosis, indicating that active nuclear import can occur during interphase in dividing cells (Fig. 7). These results again indicate that nuclear import of ASV DNA is not limited to cells undergoing mitosis; rather, our results suggest an active import process whereby trafficking of the preintegration complex to the nucleus is not temporally or physically restricted to mitosis in dividing cells. As mitosis only comprises a small percentage of the cell cycle (<10%), such a mitosis-independent pathway would suggest a more efficient import process in dividing cells than is generally believed to exist. However, we note that these results do not exclude nuclear capture of ASV DNA after mitosis.
Lastly, we measured transduction in terminally differentiated cells that are withdrawn from the cell cycle (G0). We observed that mouse hippocampal neuron explants could be transduced efficiently by ASV (Fig. 8). Paradoxically, it was previously shown that ASV reverse transcription is deficient in quiescent chicken embryo fibroblasts arrested in G0 (14). It has been generally believed that terminally differentiated cells cannot reenter the cell cycle. Recent studies indicate that neurons have the potential to reenter the cell cycle and that reentry into S phase (without cytokinesis) is associated with neuronal cell death (54). It is possible that a subset of explanted postmitotic neurons maintain proliferative potential and that they are able to support early events in ASV infection. Transduction of neurons by HIV-1 vectors appears to be limited by deoxyribonucleotide levels (2); the fresh embryonic neuron explants described here may provide a threshold level to support ASV reverse transcription.
The results presented here support the idea that ASV encodes determinants that mediate nuclear import of viral DNA. A likely candidate for at least one of these determinants is the ASV IN (25, 26). ASV IN encodes a functionally defined, noncanonical NLS. Single amino acid substitutions in the NLS cause a significant delay in viral replication without compromising IN biochemical activity in vitro (26). Multiple substitutions in the NLS are lethal for viral replication (unpublished observations). Using a permeabilized-cell assay, we have partially characterized the import pathway utilized by the ASV IN NLS. The pathway involves the nuclear pore but is apparently novel in terms of energy requirements and receptors (Andrake et al., unpublished data). We are currently investigating whether this pathway is required for ASV transduction of arrested cells. Recent studies indicate that amino acid substitutions in a putative HIV-1 IN NLS affect HIV-1 (3).
The relative efficiencies of transduction by HIV-1-, MuLV-, and ASV-based vectors were recently compared in aphidicolin-arrested human cells (18). The transduction efficiency of the ASV vector was found to be greater than that of the MuLV vector. However, it is well-established that transduction by ASV vectors in differentiated adult mouse tissues is inefficient; therefore, the utility of such vectors is thought to be limited (28). The variables that influence ASV transduction in such tissues are still unknown. It is important to consider that the efficiency of transduction (as measured by reporter expression) is determined by the efficiency of a number of individual steps, including reverse transcription, nuclear import, integration, reporter gene expression, and silencing of the integrated DNA. It has been generally believed that the basis of observed transduction differences between lentiviral (i.e., HIV-1) and oncoretroviral (MuLV) vectors relates to nuclear import functions. Our results indicate that assumptions regarding a mitosis-dependent import of ASV DNA are incorrect. We are currently investigating the biochemical pathway of DNA import and the role of the IN-NLS. Further study of the cell and tissue variables that affect retroviral transduction efficiencies should provide insights of significant value in the development and use of these vectors for laboratory and possibly gene therapy purposes.
Acknowledgments
We thank Pat Roat and Kim Boland for excellent technical assistance and Marie Estes for preparing the manuscript. We also thank Susan Shinton from the Fox Chase Cancer Center Flow Cytometry and Cell Sorting Facility for help with FACS analysis and Jonathan Boyd from the Fox Chase Cancer Center Cell Imaging Facility for assistance with microscopy. Kelly Gravuer and Heidi Harrington made valuable contributions during the early stages of this work. René Daniel and Mark Andrake provided helpful discussions, and John Taylor and Bill Mason provided critical reviews of the manuscript. We are also grateful to S. Hughes, C. Cepko, E. M. Bradbury, and M. Roth for providing reagents.
This work was supported by National Institutes of Health grants AI40385, CA71515, CA06927 (A.M.S.), and MH56951 (G.F.R.) and also by an appropriation from the Commonwealth of Pennsylvania.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or any other sponsoring organization.
REFERENCES
- 1.Barsov, E. V., and S. H. Hughes. 1996. Gene transfer into mammalian cells by a Rous sarcoma virus-based retroviral vector with the host range of the amphotropic murine leukemia virus. J. Virol. 70:3922-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomer, U., L. Naldini, T. Kafri, D. Trono, I. M. Verma, and F. H. Gage. 1997. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 71:6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky, M. I., N. Sharova, M. P. Dempsey, T. L. Stanwick, A. G. Bukrinskaya, S. Haggerty, and M. Stevenson. 1992. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. USA 89:6580-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin, J. M., S. H. Hughes, and H. Varmus. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 7.Cross, S. M., C. A. Sanchez, C. A. Morgan, M. K. Schimke, S. Ramel, R. L. Idzerda, W. H. Raskind, and B. J. Reid. 1995. A p53-dependent mouse spindle checkpoint. Science 267:1353-1356. [DOI] [PubMed] [Google Scholar]
- 8.Cullen, B. R. 2001. Journey to the center of the cell. Cell 105:697-700. [DOI] [PubMed] [Google Scholar]
- 9.Daniel, R., R. A. Katz, and A. M. Skalka. 1999. A role for DNA-PK in retroviral DNA integration. Science 284:644-647. [DOI] [PubMed] [Google Scholar]
- 10.Deminie, C. A., and M. Emerman. 1994. Functional exchange of an oncoretrovirus and a lentivirus matrix protein. J. Virol. 68:4442-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerman, M. 2000. Learning from lentiviruses. Nat. Genet. 24:8-9. [DOI] [PubMed] [Google Scholar]
- 12.Flint, S. J., L. W. Enquist, R. M. Krug, V. R. Racaniello, and A. M. Skalka. 2000. Principles of virology: molecular biology, pathogenesis, and control. ASM Press, Washington, D.C.
- 13.Fouchier, R. A., and M. H. Malim. 1999. Nuclear import of human immunodeficiency virus type-1 preintegration complexes. Adv. Virus Res. 52:275-299. [DOI] [PubMed] [Google Scholar]
- 14.Fritsch, E. F., and H. M. Temin. 1977. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J. Virol. 24:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, W. Y., A. Cara, R. C. Gallo, and F. Lori. 1993. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc. Natl. Acad. Sci. USA 90:8925-8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulaouic, H., F. Subra, J. F. Mouscadet, S. Carteau, and C. Auclair. 1994. Exogenous nucleosides promote the completion of MoMLV DNA synthesis in G0-arrested Balb c/3T3 fibroblasts. Virology 200:87-97. [DOI] [PubMed] [Google Scholar]
- 17.Harel, J., E. Rassart, and P. Jolicoeur. 1981. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology 110:202-207. [DOI] [PubMed] [Google Scholar]
- 18.Hatziioannou, T., and S. P. Goff. 2001. Infection of nondividing cells by Rous sarcoma virus. J. Virol. 75:9526-9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu, T. W., and J. M. Taylor. 1982. Effect of aphidicolin on avian sarcoma virus replication. J. Virol. 44:493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries, E. H., C. Glover, and M. E. Reichmann. 1981. Rous sarcoma virus infection of synchronized cells establishes provirus integration during S-phase DNA synthesis prior to cellular division. Proc. Natl. Acad. Sci. USA 78:2601-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung, D. T., T. F. Jamison, and S. L. Schreiber. 1996. Understanding and controlling the cell cycle with natural products. Chem. Biol. 3:623-639. [DOI] [PubMed] [Google Scholar]
- 22.Kootstra, N. A., B. M. Zwart, and H. Schuitemaker. 2000. Diminished human immunodeficiency virus type 1 reverse transcription and nuclear transport in primary macrophages arrested in early G1 phase of the cell cycle. J. Virol. 74:1712-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korin, Y. D., and J. A. Zack. 1999. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korin, Y. D., and J. A. Zack. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kukolj, G., K. S. Jones, and A. M. Skalka. 1997. Subcellular localization of avian sarcoma virus and human immunodeficiency virus type 1 integrases. J. Virol. 71:843-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kukolj, G., R. A. Katz, and A. M. Skalka. 1998. Characterization of the nuclear localization signal in the avian sarcoma virus integrase. Gene 223:157-163. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence, D. M., C. E. Patterson, T. L. Gales, J. L. D'Orazio, M. M. Vaughn, and G. F. Rall. 2000. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J. Virol. 74:1908-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis, B. C., N. Chinnasamy, R. A. Morgan, and H. E. Varmus. 2001. Development of an avian leukosis-sarcoma virus subgroup A pseudotyped lentiviral vector. J. Virol. 75:9339-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis, P., M. Hensel, and M. Emerman. 1992. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 11:3053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, G., M. Simm, M. J. Potash, and D. J. Volsky. 1993. Human immunodeficiency virus type 1 DNA synthesis, integration, and efficient viral replication in growth-arrested T cells. J. Virol. 67:3969-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, L., J. M. Olvera, K. E. Yoder, R. S. Mitchell, S. L. Butler, M. Lieber, S. L. Martin, and F. D. Bushman. 2001. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 20:3272-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lori, F., and J. Lisziewicz. 2000. Rationale for the use of hydroxyurea as an anti-human immunodeficiency virus drug. Clin. Infect. Dis. 30(Suppl. 2):S193-S197. [DOI] [PubMed] [Google Scholar]
- 34.Matise, M. P., W. Auerbach, and A. L. Joyner. 2000. Production of targeted embyonic stem cell clones, p. 101-132. In A. L. Joyner (ed.), Gene targeting: a practical approach, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 35.Meyerhans, A., J. P. Vartanian, C. Hultgren, U. Plikat, A. Karlsson, L. Wang, S. Eriksson, and S. Wain-Hobson. 1994. Restriction and enhancement of human immunodeficiency virus type 1 replication by modulation of intracellular deoxynucleoside triphosphate pools. J. Virol. 68:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, D. G., M. A. Adam, and A. D. Miller. 1990. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 10:4239-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake, K., N. Suzuki, H. Matsuoka, T. Tohyama, and T. Shimada. 1998. Stable integration of human immunodeficiency virus-based retroviral vectors into the chromosomes of nondividing cells. Hum. Gene Ther. 9:467-475. [DOI] [PubMed] [Google Scholar]
- 38.Naldini, L., U. Blömer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 39.Park, F., K. Ohashi, W. Chiu, L. Naldini, and M. A. Kay. 2000. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat. Genet. 24:49-52. [DOI] [PubMed] [Google Scholar]
- 40.Pieroni, L., P. Bouille, C. Auclair, J. J. Guillosson, and J. Nafziger. 1999. Early steps of replication of Moloney murine leukemia virus in resting lymphocytes. Virology 262:408-415. [DOI] [PubMed] [Google Scholar]
- 41.Pospiech, H., A. K. Rytkonen, and J. E. Syvaoja. 2001. The role of DNA polymerase activity in human non-homologous end joining. Nucleic Acids Res. 29:3277-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pressacco, J., J. S. Wiley, G. P. Jamieson, C. Erlichman, and D. W. Hedley. 1995. Modulation of the equilibrative nucleoside transporter by inhibitors of DNA synthesis. Br. J. Cancer 72:939-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiser, J., G. Harmison, S. Kluepfel-Stahl, R. O. Brady, S. Karlsson, and M. Schubert. 1996. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc. Natl. Acad. Sci. USA 93:15266-15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roe, T.-Y., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaefer-Klein, J., I. Givol, E. V. Barsov, J. M. Whitcomb, M. VanBrocklin, D. N. Foster, M. J. Federspiel, and S. H. Hughes. 1998. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology 248:305-311. [DOI] [PubMed] [Google Scholar]
- 46.Sutton, R. E., M. J. Reitsma, N. Uchida, and P. O. Brown. 1999. Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J. Virol. 73:3649-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tai, A. W., E. J. Lien, M. M. Lai, and T. A. Khwaja. 1984. Novel N-hydroxyguanidine derivatives as anticancer and antiviral agents. J. Med. Chem. 27:236-238. [DOI] [PubMed] [Google Scholar]
- 48.Th'ng, J. P., P. S. Wright, J. Hamaguchi, M. G. Lee, C. J. Norbury, P. Nurse, and E. M. Bradbury. 1990. The FT210 cell line is a mouse G2 phase mutant with a temperature-sensitive CDC2 gene product. Cell 63:313-324. [DOI] [PubMed] [Google Scholar]
- 49.Uchida, N., R. E. Sutton, A. M. Friera, D. He, M. J. Reitsma, W. C. Chang, G. Veres, R. Scollay, and I. L. Weissman. 1998. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 95:11939-11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varmus, H. E., T. Padgett, S. Heasley, G. Simon, and J. M. Bishop. 1977. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell 11:307-319. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg, J. B., T. J. Matthews, B. R. Cullen, and M. H. Malim. 1991. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 174:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zack, J. A. 1995. The role of the cell cycle in HIV-1 infection. Adv. Exp. Med. Biol. 374:27-31. [DOI] [PubMed] [Google Scholar]
- 53.Zack, J. A., A. M. Haislip, P. Krogstad, and I. S. Y. Chen. 1992. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J. Virol. 66:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, X., A. K. Raina, and M. A. Smith. 1999. Cell cycle events in neurons. Proliferation or death? Am. J. Pathol. 155:327-329. [DOI] [PMC free article] [PubMed] [Google Scholar]