Abstract
The tegument protein pp71 (UL82) of human cytomegalovirus (HCMV) has previously been shown to transactivate the major immediate-early enhancer-promoter of HCMV. Furthermore, this protein is able to enhance the infectivity of viral DNA and to accelerate the infection cycle, suggesting an important regulatory function during viral replication. To gain insight into the underlying mechanisms that are used by pp71 to exert these pleiotropic effects, we sought for cellular factors interacting with pp71 in a yeast two-hybrid screen. Here, we report the isolation of the human Daxx (hDaxx) protein as a specific interaction partner of HCMV pp71. hDaxx, which was initially described as an adapter protein involved in apoptosis regulation, has recently been identified as a nuclear protein that interacts and colocalizes with PML in the nuclear domain ND10. In order to assess whether pp71 can also be detected in ND10 structures, a vector expressing pp71 in fusion with the green fluorescent protein was used for transfection of human fibroblasts. This revealed a colocalization of pp71 with the ND10 proteins PML and Sp100. In addition, cotransfection of a hDaxx expression vector resulted in an enhanced recruitment of pp71 to ND10. Targeting of pp71 to nuclear dots could also be observed in infected human fibroblasts in the absence of de novo viral protein synthesis. Moreover, cotransfection experiments revealed that pp71-mediated transactivation of the major immediate-early enhancer-promoter was synergistically enhanced in the presence of hDaxx. These results suggest an important role of hDaxx for pp71 protein function.
Human cytomegalovirus (HCMV), a member of the beta-subgroup of herpesviruses, is characterized by its narrow host range and prolonged replicative cycle in cell culture, as well as in the infected human host. Generally, HCMV causes asymptomatic infections in immunocompetent individuals; severe disease, however, can result from infection in immunocompromised patients and after intrauterine infection (4).
Similar to other herpesviruses, the HCMV open reading frames are expressed in a temporally regulated cascade consisting of three sequential phases, termed immediate early (IE), early (E) and late (L) (19, 27, 44, 47, 69, 70). IE gene expression results in the synthesis of viral regulatory factors, in particular the major IE proteins IE1-p72 and IE2-p86, which act as strong transactivators of viral early promoters and are therefore required for efficient productive infection (44, 51, 53, 65). Expression from the major IE gene locus is driven by a very strong, complex regulatory element known as the major IE enhancer-promoter (MIEP), which contains several binding sites for known eucaryotic transcription factors (for a review, see reference 48). These in turn modulate gene expression not only in various cell types but also in both differentiated and undifferentiated cells (6, 12, 23, 43, 48, 50, 60, 63). In addition to cellular transcription factors, activation of IE transcription is controlled by structural protein components of the incoming virion (62, 66). These proteins are localized in the so-called tegument, which separates the nucleocapsid from the viral envelope. Tegument components are often phosphoproteins which are thought to have both structural and regulatory functions. In the case of the alphaherpesvirus herpes simplex virus type 1 (HSV-1), the tegument protein VP16 in complex with two cellular factors is responsible for stimulation of the IE phase (for a review, see reference 57). Concerning HCMV, at least 20 proteins of both cellular and viral origin are estimated to be part of the tegument. However, only some of these tegument proteins have already been investigated in greater detail, such as the viral phosphoproteins ppUL69, pp65, and pp71 (10, 24, 25, 52, 56, 58, 61, 74). The most abundant protein of the tegument is the so-called lower matrix protein pp65, which is expressed from the reading frame UL83. From the adjacent gene locus, UL82, the upper matrix protein pp71 is synthesized. This protein is discussed as the HCMV functional analogue of the HSV-1 VP16 transactivator since pp71 is able to activate IE gene expression in the infected cell via stimulation of the HCMV MIEP, as well as additional viral and cellular promoters (15, 17, 34, 42, 74). Consistent with these findings, it was demonstrated that pp71 is imported into the nucleus immediately after infection (30). Furthermore, pp71 has been shown to enhance the infectivity of viral DNA and to accelerate the viral infectious cycle (9).
The specific mechanism of how pp71 activates transcription is poorly understood. As is the case for HSV-1 VP16, additional cellular factors are discussed to contribute to pp71-mediated transactivation, since no direct binding of pp71 to DNA has been detected thus far (42). Therefore, we were looking for potential cellular binding partners of pp71 by yeast two-hybrid screening. Hereby, we identified the factor hDaxx as specific interaction partner of pp71. This cellular protein has been shown to be localized within specific subnuclear compartments known as ND10 domains, which in turn are the transcriptional active sites during HCMV IE gene expression (35, 36, 45). We provide evidence here that this interaction is required for colocalization of pp71 with ND10 domains both after transient transfection and viral infection. Furthermore, pp71 is recruited to the ND10 domain by hDaxx, which in turn results in an enhanced transactivation capacity of pp71.
(Initial reports of this work were presented at The 25th International Herpesvirus Workshop [Portland, Oreg., July 29 to August 4, 2000].)
MATERIALS AND METHODS
Oligonucleotides.
Oligonucleotides were obtained from Eurogentec (Seraing, Belgium) and ARK (Darmstadt, Germany). The following oligonucleotides (5′-to-3′ sequences) were used for cloning and site-directed mutagenesis: pp71-5NheI, GCTCGGATCCTCATATGTCTCAGGCATCGTCCTCGC; pp71-3BamHI, AACAGGATCCTCTCTCTAGATGCGGGGTCG; pp71pEGFP-N1-5, ATGGAGCTCATGTCTCAGGCATCGTCCTCG; pp71pEGFP-N1-3, ATGGAATTCTGCGGGGTCGACTGCGTGG; pp71-3XbaI, ATGTCTAGACTAGATGCGGGGTCGACTGCG; myc-pp71-5′, AGTCGGATCCATGTCTCAGGCATCGTCCTCG; pp71-DID1-3, AGTCGAATTCGATGGCGCCGGCGCGAAAGGC; pp71-DID1-5, AGTCGAATTCGAGCAGCTGGCCTGTTCGG; pp71-DID2-3, AGTCGAATTCGATGTTTTCCGGGAAAAAGATGG; pp71-DID2-5, AGTCGAATTCCCGCTACCCGATCGTGTGCG; myc-5′pcDNA3, AGCTTCGCCACCATGGAACAAAAACTCATCTCAGAAGAGGATCTGG; myc-3′pcDNA3, GTACCCAGATCCTCTTCTGAGATGAGTTTTTGTTCCATGGTG GCGA; FL-Sp100-5′, AGTCAGAATTCTGATGGCAGGTGGGGGCGGC; FL-Sp100-3′, AGTCGATATCCTAATCTTCTTTACCTGACCC; DAXXaa371-5′, CATAGGATCCGGCTGGATGAGGTCATCTC; DAXXaa197-5′, CATAGGATCCTCTATGTGGCAGAGATCCG; DAXXaa43-5′, CATAGGATCCCTCATGGGGCCAGAGGAAG; DAXXaa740-3′: CATAGTCGACCTAATCAGAGTCTGAGAGC; DAXXaa657-3′, CATAGTCGACCTCATGCACTGACCTTTGCC; DAXXaa628-3′, CATAGTCGACGGGGGGACCAGAATCTCCCC; DAXXaa560-3′, CATAGTCGACTTCTTCCTCCAGGGTCAGCT; DAXXaa501-3′, CATAGTCGACCTGTAGTGAGGACATGGGGC; DAXXaa439-3′, CATAGTCGACGTCCTCGTCATCTGTCTCAGC; DAXXaa371-3′, CATAGTCGACCCGACTCATGGCCAAACTCCG; pp71mutP206A-5, CGCGCCGGCGCCATCGCACTGACGCTGGTAGAC; pp71mutP206A-3, GTCTACCAGCGTCAGTGCGATGGCGCCGGCGCG; pp71mutL207A-5, GCCGGCGCCATCCCGGCCACGCTGGTAGACGCC; pp71mutL207A-3, GGCGTCTACCAGGCTGGCCGGGATGGCGCCGGC; pp71mutV210A-5, ATCCCGCTGACGCTGGCCGACGCCCTGGAGCAG; pp71mutV210A-3, CTGCTCCAGGGCGTCGGCCAGCGTCAGCGGGAT; pp71mutP324A-5, CCCGGAAAACATCGCAGGCGTCTCCATAGAAGCC; pp71mutP324A-3, GGCTTCTATGGAGACGCCTGCGATGTTTTCCGGG; pp71mutV326A-5, CCGGAAAACATCCCGGGCGCCTCCATAGAAGCCGGC; pp71mutV326A-3, GCCGGCTTCTATGGAGGCGCCCGGGATGTTTTCCGG; pp71mutI328A-5, ATCCCGGGCGTCTCCGCCGAAGCCGGCCCGCTA; pp71mutI328A-3: TAGCGGGCCGGCTTCGGCGGAGACGCCCGGGAT; pp71mutP332A-5, CTCCATAGAAGCCGGCGCACTACCCGATCGTGTG; and pp71mutP332A-3, CACTCGATCGGGTAGTGCGCCGGCTTCTATGGAG.
Plasmid constructions and in vitro mutagenesis.
The bait plasmid pHM677 for the yeast two-hybrid screen was constructed by PCR amplification with the oligonucleotides pp71-5NheII and pp71-3BamHI and plasmid pCB6-pp71 containing the pp71 cDNA as a template (30), followed by insertion into the GAL4 DNA-binding domain vector pAS1 via NheI and BamHI (20). Deletion mutagenesis of hDaxx in the context of the GAL4 activation domain vector pGAD424 (Clontech, Palo Alto, Calif.) was performed by PCR mutagenesis with the 5′oligonucleotides DAXXaa43-5, DAXXaa197-5, and DAXXaa371-5 in combination with the 3′oligonucleotides DAXXaa740-3, DAXXaa657-3, DAXXaa628-3, DAXXaa560-3, DAXXaa501-3, DAXXaa439-3, and DAXXaa371-3, respectively. After cleavage with BamHI and SalI, the PCR products were cloned into the pGAD424 vector. The yeast vectors pAS-PML and pAS-Sp100 were a kind gift of G. Maul (Philadelphia, Pa.). The plasmid UL69-pGBT was described earlier (72); the vector UL26-pGBT will be described in detail elsewhere.
For expression in mammalian cells, the following plasmids were constructed. A plasmid expressing the pp71-green fluorescent protein (GFP) fusion protein was created by PCR amplification of the pp71 open reading frame by using oligonucleotides pp71pEGFP-N1-5 and pp71pEGFP-N1-3, followed by cleavage with SstI and EcoRI and ligation with the vector pEGPF-N1 (Clontech). An expression vector for myc-tagged proteins was constructed by annealing oligonucleotides myc-5′pcDNA3 and myc-3′pcDNA3, followed by insertion into the pcDNA3 vector (Invitrogen, San Diego, Calif.) via the HindIII and Asp718 sites, resulting in plasmid myc-pcDNA3. The myc-pp71 expression vector was thereafter constructed by amplification of the pp71 open reading frame by using oligonucleotide myc-pp71-5′ and pp71-3 XbaI. The resulting PCR product was cleaved with BamHI and XbaI and ligated with the myc-pcDNA3 vector. The internal pp71 deletion mutants pp71Δ(206-213) and pp71Δ(324-331) were constructed by PCR amplification of the N-terminal pp71 fragments by using the oligonucleotide myc-pp71-5′ in combination with pp71-DID1-3 or pp71-DID2-3, respectively. The C-terminal pp71 fragments were amplified by using the oligonucleotides pp71-DID1-5 or pp71-DID2-5, as well as oligonucleotide pp71-3XbaI. Thereafter, the N-terminal fragments were inserted into the myc-pcDNA3 vector by using the BamHI and EcoRI restriction sites, followed by ligation with the C-terminal fragment via EcoRI and XbaI. Further subcloning of these internal deletion mutants into the yeast vector pAS1 and vector pEYFP-C1 was performed in analogy to wild-type pp71 as described above. Site-directed mutagenesis was performed by using the QuikChange Site-Directed Mutagenesis Kit according to the manufacturer's protocol (Stratagene, Heidelberg, Germany) with either the myc-pp71 plasmid or the pp71-GFP vector as templates. The pp71 single-amino-acid mutants were constructed by using the respective oligonucleotides mentioned above. The double-amino-acid mutant pp71-P324A/P332A was created by using the pp71-P324A plasmid as a template and oligonucleotides pp71mutP332A-5 and pp71mutP332A-3; the double-amino-acid mutant pp71-V326A/P332A was generated by PCR mutagenesis of vector pp71-V326A and oligonucleotides pp71mutP332A-5 and pp71mutP332A-3. The double-amino-acid mutant L207A/T208S was produced due to a second-site mutation within oligonucleotides pp71mutL207A-5 and pp71mutL207A-3.
The FLAG-hDaxx expression vector was created by isolation of the hDaxx insert from one of the respective library clones obtained in the yeast two-hybrid screen and ligation with the FLAG-pcDNA3 vector by using the EcoRV and XbaI sites (32). N- and C-terminal hDaxx deletion mutants were isolated from the respective yeast expression vectors via BamHI and SalI and ligated with the FLAG-pcDNA3 vector additionally encoding the simian virus 40 nuclear localization signal to ensure nuclear localization. A plasmid expressing FLAG-Sp100 was constructed by PCR amplification of the Sp100 cDNA from vector pSG-Sp100 (kindly provided by T. Sternsdorf, Hamburg, Germany) with oligonucleotides FL-Sp100-5′and FL-Sp100-3′; the resulting PCR product was restricted with EcoRI and EcoRV and ligated with the FLAG-pcDNA3 vector. Plasmids encoding FLAG-hSPT6, FLAG-IE2 (amino acids 135 to 579) and pCB6-UL69 were described previously (32, 72, 73). The luciferase reporter construct containing the HCMV major IE enhancer-promoter, termed pHM287, has also been described previously (73). The DNA sequence of all plasmid constructs was confirmed by automated sequence analysis (ABI, Weiterstadt, Germany).
Yeast two-hybrid screening.
Yeast two-hybrid screening was performed with GAL4 fusion proteins as described previously (18, 21). Yeast strain Y153 was transformed by the lithium acetate method by using the bait plasmid pHM677 (26). The presence of pHM677 in the yeast cells was maintained stable by selection for tryptophane prototrophy. Expression of the resulting pp71 fusion protein GAL4-pp71 was confirmed by Western blot analysis with an anti-His antiserum. Y153 yeast cells transfected with pHM677 alone or in combination with pACT were then tested for activation of the reporter genes HIS3 and lacZ. In neither case was an activation of the reporter genes by GAL4-pp71 observed. Yeast strain Y153 containing pHM677 was subsequently transformed with a cDNA library derived from human B lymphocytes fused to the GAL4 activation domain in the pACT vector (20). A total of 107 primary transformants were selected for growth on histidine dropout plates containing 20 mM 3-aminotriazole. His+ colonies were subsequently analyzed for β-galactosidase activity by filter test experiments (13). Interactor plasmids from positive clones were rescued by transformation of competent KC8 bacteria with total yeast DNA (31). The nucleotide sequences of the cDNA inserts were determined by automated sequence analysis (ABI).
Cell culture, virus infection, transfection, and reporter assays.
Human foreskin fibroblast (HFF) cells were cultured as described previously (64). U373MG and 293 cells were obtained from the American Type Culture Collection (Rockville, Md.) and maintained in Dulbecco minimal essential medium (Gibco-BRL, Eggenstein, Germany) supplemented with 5% or 10% fetal calf serum, respectively. The day before transfection, HFF cells were plated onto six-well plates at 3.5 × 105 cells per well. 293 cells were seeded onto six-well plates at 3 × 105 cells per well 2 days before transfection. Plasmid transfections were performed by the calcium phosphate coprecipitation procedure by using BES [N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid] and a total of 1 to 2 μg of DNA for six-well plates (8). Cells were harvested 48 h after transfection and used for Western blotting or immunoprecipitation. For indirect immunofluorescence analysis, HFF cells grown on coverslips were transfected with 2 μg of plasmid DNA by using the FuGENE transfection reagent according to the manufacturer's protocol (Boehringer Mannheim, Mannheim, Germany). For luciferase assays, U373MG cells were plated onto six-well dishes at 3.5 × 105 cells per well the day before transfection. Plasmid transfection was performed by the DEAE-dextran method as described previously (7). Routinely, 1 μg of luciferase target and 4.5 μg of the cotransfected transactivator plasmids were used. The total amount of transfected DNA was kept constant by using the cloning vectors pCB6, FLAG-pcDNA3, or myc-pcDNA3 in order to replace the missing transactivator plasmid. Approximately 48 h after transfection, cells were harvested and luciferase assays were performed as described previously (32). Luciferase activity in the supernatant was determined by using a luminometer (Bertholt, Freiburg, Germany). Each transfection was performed in triplicates and was repeated at least three times. Infection of HFF cells with HCMV (0.5 PFU/cell) and treatment of cells with cycloheximide were performed as described previously (55, 64).
Western blotting and immunoprecipitation analysis.
Coimmunoprecipitation analysis for detection of protein interactions was performed as described previously (11). Briefly, transfected cells were lysed in NP-40 lysis buffer for 20 min at 4°C, followed by a high-speed centrifugation. The supernatant was incubated with the appropriate antibody for 1 h at 4°C, and then a 50% protein A-Sepharose solution was added for another 2 h. The Sepharose beads were collected and washed three times in NP-40 lysis buffer. Antigen-antibody complexes were recovered by boiling in sodium dodecyl sulfate (SDS) sample buffer. Samples were subjected to SDS-10% polyacrylamide gel electrophoresis (PAGE), and the proteins were transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Western blotting and chemiluminescence detection were performed according to the manufacturer's protocol (ECL Western detection kit; Amersham Pharmacia Biotech Europe, Freiburg, Germany).
Antibodies and indirect immunofluorescence analysis.
Monoclonal antibody anti-FLAG M2, which is directed against the synthetic FLAG octapeptide N-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-C, was purchased from INTEGRA Bioscience (Fernwald, Germany). Hybridoma cells producing monoclonal antibody 9E10 against the synthetic myc epitope N-Met-Glu-Gln-Lys-Leu-Ile-Ser-Glu-Glu-Asp-Leu-C were a kind gift of S. Lang (Erlangen, Germany). For detection of endogenous ND10 proteins, polyclonal antibody M-112 against hDaxx and monoclonal antibody PG-M3 against PML were employed (Santa Cruz Biotechnology, Santa Cruz, Calif.). The anti-Sp26 antiserum directed against Sp100 was kindly provided by T. Sternsdorf (Hamburg, Germany). The monoclonal and polyclonal antibodies recognizing ppUL69 or IE2-p86 of HCMV were described previously (22, 73). The monoclonal antibody MAb810 against HCMV major IE proteins was obtained from Chemicon (Hofheim, Germany) (46). The monoclonal antibody p63-27 directed against IE1 was described previously (5). Monoclonal antibodies 65-33 (directed against pp65) and CMV 355 (directed against pp71) were the kind gifts of B. Britt and T. Shenk, respectively. Anti-mouse and anti-rabbit horseradish peroxidase- or fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were obtained from Dianova (Hamburg, Germany).
For indirect immunofluorescence analysis, HFF cells on coverslips were washed two times with phosphate-buffered saline (PBS), followed by fixation with 4% paraformaldehyde for 15 min at room temperature. Cells were permeabilized with PBS-0.2% Triton X-100 on ice for 20 min. Thereafter, the cells were incubated for 30 min at 37°C with primary antibodies in PBS, followed by incubation with FITC- and TRITC (tetramethyl rhodamine isocyanate)-conjugated secondary antibodies at a dilution of 1/100 in PBS. Cells were mounted by using Vectashield mounting medium plus DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories, Burlingame, Calif.) and analyzed by using a Zeiss Axiovert-135 microscope. FITC and TRITC were analyzed by using filter sets 10 and 14 (Zeiss, Jena, Germany) with excitation wavelengths of 450 to 490 nm and 510 to 560 nm, respectively. In the case of double staining, the two channels were recorded when no cross talk was detectable, and the channels were overlaid by the computer for dual images. Images were recorded with a cooled Spot Color digital camera (Diagnostic Instruments, Burroughs, Mich.) and processed by using the Meta-Imaging series and Adobe Photoshop (Universal Imaging Corp., Brandywine, Pa.; Adobe Systems, Inc.). Because of the variability of protein expression after transient transfection, at least 50 cells were studied in each sample. Each experiment was repeated at least three times and evaluated by two investigators.
RESULTS
Yeast two-hybrid experiments identify the cellular factor hDaxx as a strong binding partner of HCMV pp71.
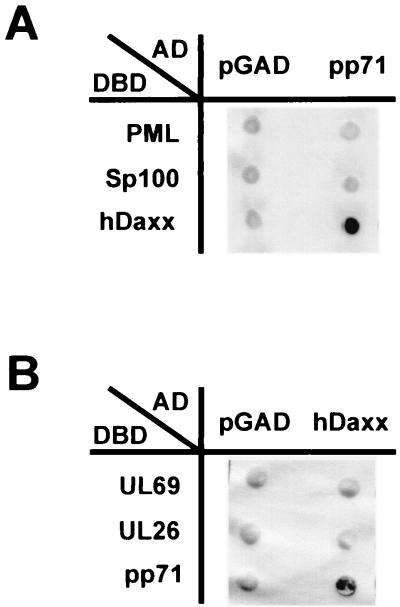
To identify novel cellular binding partners of the HCMV tegument protein pp71, a yeast two-hybrid screen was carried out. For this, we used the full-length pp71 protein as fusion with the GAL4 DNA-binding domain in the pAS vector (pHM677) as a bait. The presence of this plasmid in yeast strain Y153 was maintained stable by selection in liquid culture medium lacking tryptophane, and the expression of the pp71-GAL4 fusion protein was confirmed by Western blot analysis (data not shown). Furthermore, we confirmed that expression from this construct did not activate the reporter genes lacZ and HIS3 in yeast in the absence of an interacting protein (Fig. 1).
FIG. 1.
Specific interaction between HCMV pp71 and the hDaxx protein in yeast cells. Yeast cells were transformed with two separate vectors, one of which encoded a GAL4 activation domain either alone (pGAD) or as fusion with the indicated protein (AD), the second plasmid encoded a GAL4 DNA-binding domain fusion as indicated (DBD). Thereafter, yeast colonies were selected for the presence of both plasmids on dropout medium lacking tryptophane and leucine and subsequently analyzed for the expression of β-galactosidase. (A) Interaction analysis between pp71 fused to the GAL4 activation domain and the ND10-associated factors PML, Sp100, and hDaxx as fusions with the GAL4 DNA-binding domain. (B) Interaction analysis between hDaxx fused to the GAL4 activation domain and the HCMV tegument proteins UL69, UL26, and pp71 as fusions with the GAL4 DNA-binding domain.
The yeast two-hybrid screen was performed by transformation of yeast strain Y153 containing plasmid pHM677 (Y153/pHM677) with a cDNA library from human B lymphocytes, which is highly complex and has already been used for the successful identification of interacting proteins (20, 32, 72). We analyzed 2.5 × 107 primary transformants for interaction with pp71 by selection for histidine prototrophy on dropout plates supplemented with 20 mM 3-aminotriazole and for expression of β-galactosidase as determined by filter lift tests. Thereafter, plasmids encoding putative binding partners of pp71 were isolated from double-positive yeast clones and retransformed into Y153/pHM677 in order to confirm the interaction. Positive clones after retransformation were then subjected to automated sequencing and homology searches in the National Center for Biotechnology Information databases. In total, we identified 25 putative cellular binding partners of pp71. Most of them were isolated in several copies, indicating a sufficient complexity of the cDNA library and the specificity of the interaction with pp71. We concentrated here on the interaction between pp71 and the human Daxx protein (hDaxx) since this interaction appeared to be very strong in the yeast system.
hDaxx was originally cloned as an adapter molecule binding to the death domain of Fas (75). It was also reported to interact with the CENP-C protein and the transcription factors Ets-1 and Pax-3, resulting in transcriptional repression (33, 41, 54). Furthermore, hDaxx turned out to bind to the ND10 protein PML and, as a consequence, to colocalize with PML within these subnuclear compartments (35, 40). Since hDaxx has been identified as an interactor of various cellular proteins, we wondered whether binding to pp71 was specific. For that reason, we performed several control experiments in yeast: first, we confirmed that pp71 was not capable of binding the ND10 proteins PML and Sp100 (Fig. 1A); similarly, hDaxx was not able to interact with pUL26 and ppUL69, two other HCMV tegument proteins (Fig. 1B). We concluded from these experiments that the binding between pp71 and hDaxx in the yeast two-hybrid system was specific.
Coimmunoprecipitation experiments confirm the interaction between pp71 and hDaxx.
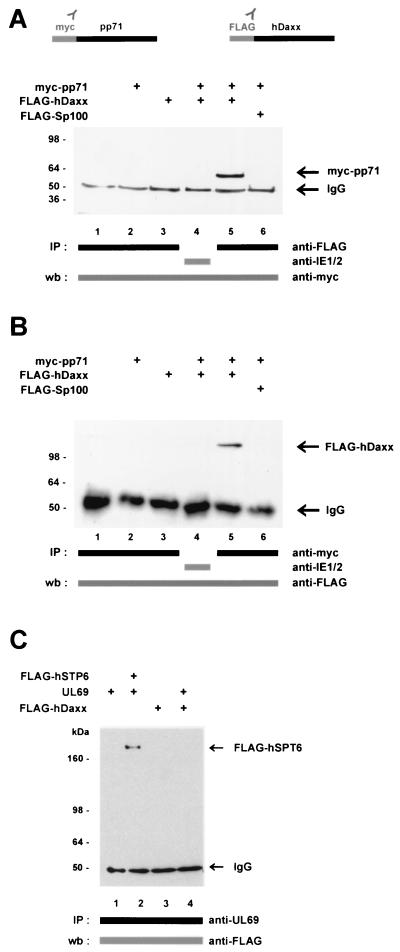
In order to confirm the data obtained in yeast, coimmunoprecipitation experiments were employed. To be able to immunologically detect pp71 and hDaxx, we fused pp71 to the myc epitope (named myc-pp71) and hDaxx to the FLAG epitope (named FLAG-hDaxx) in the context of respective eukaryotic expression vectors. Thereafter, 293 cells were transfected with these constructs either alone or in combination. Here as well as in the following experiments, an aliquot of each sample was analyzed by Western blotting prior to immunoprecipitation in order to confirm that equal amounts of protein had been expressed (data not shown). Immunoprecipitation was performed with the anti-FLAG monoclonal antibody against FLAG-tagged proteins; subsequently, coprecipitated pp71 protein was detected by Western blotting with the monoclonal anti-myc antibody (Fig. 2A). After precipitation of FLAG-hDaxx with the anti-FLAG antibody, the bound pp71 protein was clearly visible in Western blot analysis (Fig. 2A, lane 5). This was not the case when using an unrelated antibody for precipitation (Fig. 2A, lane 4) or in the absence of one interaction partner (Fig. 2A, lanes 2 and 3). Furthermore, it was excluded that pp71 was able to bind to the ND10 factor Sp100, which was also expressed as a FLAG-tagged protein (Fig. 2A, lane 6). To further confirm this observation, we performed the reciprocal experiment, in which we precipitated the pp71 fusion protein by using the anti-myc antibody and thereafter employed the anti-FLAG antibody for detection of interacting factors in Western blot (Fig. 2B). The interaction here also required a specific antibody for precipitation and the presence of both binding partners (Fig. 2B, lane 5). In order to strengthen the specificity of the pp71-hDaxx interaction, additional coimmunoprecipitation analyses were performed. As can be seen from Fig. 2C, the HCMV tegument protein ppUL69 did not interact with hDaxx (Fig. 2C, lane 4), whereas the previously described interaction between ppUL69 and the cellular protein hSPT6 (Fig. 2C, lane 2) could be confirmed under the conditions used in this experiment (72). In similiar experiments, we could also exclude binding between hDaxx and the HCMV regulator pUL84 (data not shown). Because of the results of both the yeast system and the coimmunoprecipitation experiments, we considered the interaction between pp71 and hDaxx as specific.
FIG. 2.
Specific interaction between pp71 and hDaxx as determined in coimmunoprecipitation experiments. 293 cells were transfected with eukaryotic expression vectors encoding myc-pp71, FLAG-hDaxx, and FLAG-hSPT6, as well as the HCMV protein ppUL69, as indicated and prepared for immunoprecipitation as described in Materials and Methods. Immunoprecipitation was performed with antibodies as indicated by bars. Precipitates were washed three times and separated by SDS-10% PAGE. (A) Immunoprecipitations were performed with the anti-FLAG monoclonal antibody or an anti-IE2 monoclonal antibody as indicated. Thereafter, coprecipitated pp71protein was detected in Western blot analysis with the anti-myc monoclonal antibody. Lanes: 1, lysates from untransfected cells; 2, transfection with plasmid myc-pp71 alone; 3, transfection with vector FLAG-hDaxx alone; 4 and 5, transfections with a combination of vectors encoding myc-pp71 and FLAG-hDaxx; 6, transfection with plasmid myc-pp71 and a plasmid encoding FLAG-Sp100. (B) Immunoprecipitations were performed with the anti-myc monoclonal antibody or the anti-IE2 monoclonal antibody. Interacting proteins were thereafter detected in a Western blot with the anti-FLAG monoclonal antibody. The lanes are analogous to those in panel A. (C) Immunoprecipitations were performed with the anti-UL69 monoclonal antibody; thereafter, coprecipitated proteins were detected by Western blot analysis with the anti-FLAG monoclonal antibody. The interaction between ppUL69 and the cellular protein hSPT6 served as a positive control (lane 2). Lanes: 1, transfection of expression vector pCB6-UL69; 2, transfection with a combination of plasmids encoding ppUL69 and FLAG-hSPT6; 3, transfection with plasmid FLAG-hDaxx; 4, transfection with constructs expressing FLAG-hDaxx and ppUL69. Molecular masses are indicated in kilodaltons. Abbreviations: IP, immunoprecipitation; wb, Western blot; IgG, immunoglobulin G.
The interaction with pp71 requires domains of hDaxx which are distinct from the PML binding site.
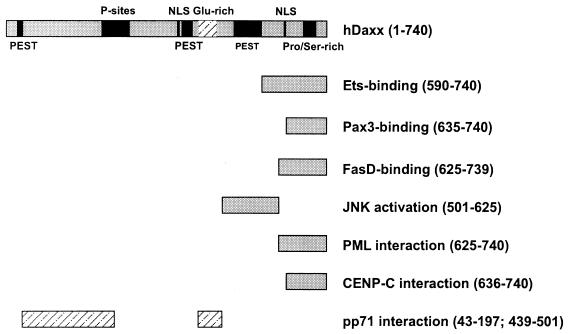
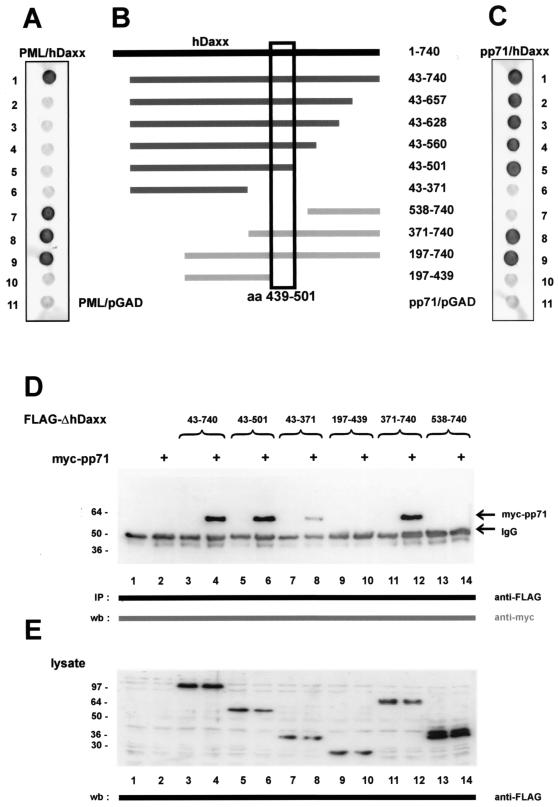
As described above, hDaxx had been reported to interact with a number of cellular proteins. All these factors have in common is that they require the hDaxx carboxy terminus for binding (see Fig. 4 for a detailed listing) (33, 40, 41, 54, 75). In order to determine which part of hDaxx was necessary for pp71 interaction, a series of hDaxx deletion mutants in fusion with the GAL4 activation domain was constructed (Fig. 3B). These mutants were transformed into yeast cells together with a vector expressing an unfused GAL4 DNA-binding domain in order to exclude activation of the respective reporter genes in the absence of an interacting factor (data not shown). Thereafter, the hDaxx mutants were tested for interaction with a PML fusion as positive control because it had already been published that the carboxy-terminal amino acids 625 to 740 within hDaxx were required for PML binding (Fig. 3A) (35, 40). We could confirm this with our deletion mutants and were able to further narrow down the PML interaction domain to amino acids 657 to 740 within hDaxx (Fig. 3A, lanes 1 and 2).
FIG. 4.
Schematic overview depicting interaction domains within hDaxx required for association with previously published cellular proteins and HCMV pp71 (33, 35, 41, 54, 75). The amino acids of hDaxx required for the respective interactions are indicated.
FIG. 3.
Delineation of the pp71 interaction domain within hDaxx. (A, B, and C) Yeast cells were transformed with two separate vectors, one of which encoded either PML as a positive control (A) or pp71 (C) fused to the GAL4 DNA-binding domain. The second plasmid encoded amino- or carboxy-terminal fragments of hDaxx as fusion with the GAL4 activation domain; the amino acids contained in the respective deletion mutants are indicated in panel B. Yeast colonies were selected for the presence of both plasmids on dropout medium lacking tryptophane and leucine and subsequently analyzed for the expression of β-galactosidase by filter lift assays. As negative controls, the activation domain vector pGAD424 (pGAD) was either transformed with the pp71 or the PML DNA-binding domain fusion (lanes 11, panels A and C, respectively). The pp71 interaction domain within hDaxx is depicted by the box. (D) Interaction between pp71 and hDaxx deletion mutants after coimmunoprecipitation from 293 cells. The hDaxx mutants were precipitated with the anti-FLAG monoclonal antibody; therafter, bound pp71 protein was detected in Western blot experiments employing the anti-myc antibody. Lanes: 1, lysates from untransfected cells; 2, transfection with plasmid myc-pp71 alone; 3, transfection with vector FLAG-hDaxx alone; 4, transfection with a combination of vectors encoding myc-pp71 and FLAG-hDaxx; 5, transfection with a plasmid encoding FLAG-hDaxx 43-501; 6, transfection with vectors encoding myc-pp71 and FLAG-hDaxx 43-501; 7, transfection with vector FLAG-hDaxx 43-371; 8, transfection with a combination of vectors myc-pp71 and FLAG-hDaxx 43-371; 9, transfection with a plasmid expressing FLAG-hDaxx 197-439; 10, transfection with vectors encoding myc-pp71 and FLAG-hDaxx 197-439; 11, transfection with plasmid FLAG-hDaxx 371-740; 12, transfection with a combination of vector myc-pp71 and FLAG-hDaxx 371-740; 13, transfection with a plasmid encoding FLAG-hDaxx 538-740; 14, transfection with vectors expressing myc-pp71 and FLAG-hDaxx 538-740. (E) Western blot analysis of the cell lysates used for immunoprecipitation in panel D. The expression of the hDaxx deletion mutants was investigated with the anti-FLAG monoclonal antibody. Molecular masses are indicated in kilodaltons. Abbreviations: IP, immunoprecipitation; wb, Western blot; IgG, immunoglobulin G.
Similiar experiments were performed with the pp71 fusion and the respective hDaxx deletion mutants (Fig. 3C). Interestingly, we could not detect an interaction between pp71 and the carboxy terminus of hDaxx which was required for interaction with the cellular factors mentioned above (Fig. 3C, lane 7). In contrast, the central glutamine-rich domain of hDaxx comprising amino acids 439 to 501 was necessary for pp71 binding (Fig. 3C, compare lanes 5, 8, and 10). We subsequently wanted to verify these findings in mammalian cells. For this purpose, we cloned the respective hDaxx deletion mutants in the FLAG-pcDNA3 vector supplied with the simian virus 40 nuclear localization signal in order to ensure nuclear localization (data not shown). After expression in 293 cells, the stability and solubilities of the mutants in NP-40 lysis buffer were examined by Western blotting (Fig. 3E); thereafter, the mutants were analyzed for pp71 interaction by coimmunoprecipitation experiments. Consistent with the findings in yeast, we observed that the glutamine-rich region within hDaxx was required for pp71 binding (Fig. 3D, lanes 6, 10, and 12), whereas the PML interaction site was completely dispensable (lane 14). Additionally, however, the hDaxx mutant comprising amino acids 43 to 371, which was defective for pp71 binding in the yeast system, also showed a weak but detectable interaction (lane 8). Therefore, two separate binding sites for pp71 appear to be present in hDaxx: one strong binding site involves the central Glu-rich region, whereas a second, weaker site is localized within the amino teminus of hDaxx. In summary, we could show that the PML interaction domain in hDaxx is dispensable for pp71 binding (see Fig. 4). This led us to the hypothesis that hDaxx might be able to bind both PML and pp71 at the same time.
Immunofluorescence analyses reveal a colocalization of pp71 with endogenous ND10 domains after transient transfection.
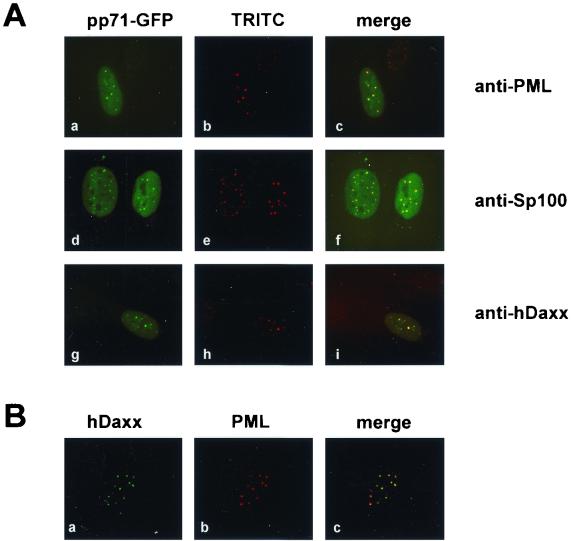
The identification of a ND10 component as binding partner of pp71 suggested that pp71 might also be present in ND10 domains as a consequence of this interaction. In order to address this question, an expression construct for pp71 fused C-terminally to GFP (pp71-GFP) was employed. After transfection of this construct into HFF cells, indirect immunofluorescence analyses were carried out. We observed a clear accumulation of pp71-GFP in nuclear speckles in addition to a diffuse nuclear staining. In order to determine whether these dots represented ND10 domains, we costained the transfected cells with antibodies detecting endogenous PML, Sp100, or hDaxx. As can be seen in Fig. 5A, pp71-GFP colocalized with all of these proteins in ND10 domains. Identical results were obtained with a construct expressing YFP fused to the N terminus of pp71 (YFP-pp71), indicating that pp71 subcellular localization was not influenced depending on whether the GFP/YFP was fused to the N- or C-terminal end of pp71 (data not shown). As high hDaxx concentrations had also been published for centromeres (54), we investigated whether hDaxx in HFF cells could also be found in regions distinct from ND10 domains. For this, we performed double immunofluorescence analyses staining endogenous hDaxx and PML, in which we detected hDaxx exclusively in ND10 in all cells examined (Fig. 5B). This observation, together with the colocalization of the pp71-fusion protein with all of the ND10 factors tested, led us to the conclusion that pp71 is able to target ND10 domains after transient transfection.
FIG. 5.
(A) Subcellular localization of pp71-GFP in HFF cells. HFF cells grown on coverslips were transfected with an expression vector encoding pp71 fused to GFP (pp71-GFP) (panels a, d, and g). In order to detect endogenous ND10 domains, indirect immunofluorescence analyses were performed with a monoclonal antibody against PML (panel b) or polyclonal antisera recognizing Sp100 (panel e) and hDaxx (panel h), followed by incubation with TRITC-conjugated anti-mouse or anti-rabbit secondary antibodies. (B) Analysis of hDaxx distribution in HFF cells. HFF cells were costained for endogenous expression of hDaxx as detected by the polyclonal antiserum (panel a) and PML with a monoclonal antibody (panel b).
pp71 is recruited to ND10 domains after coexpression of FLAG-hDaxx.
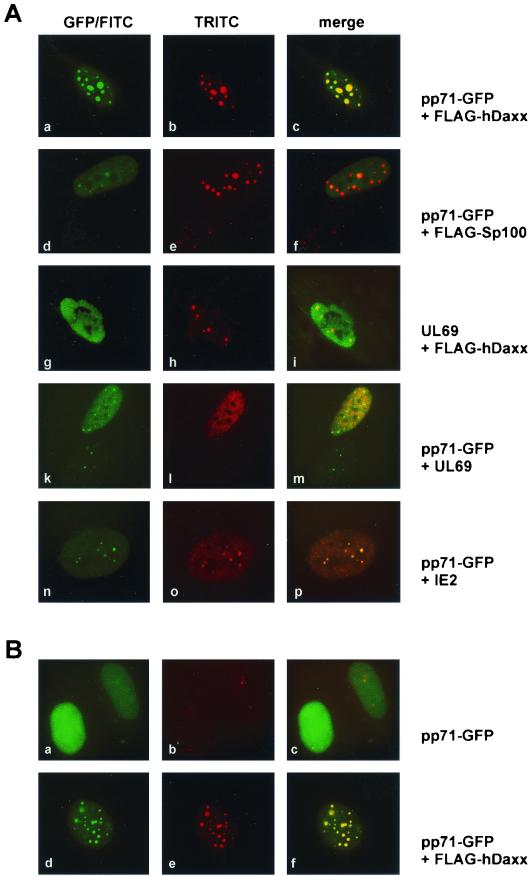
Additionally, we wanted to investigate whether coexpression of hDaxx would alter pp71 localization within the nucleus. For this, HFF cells were cotransfected with the pp71-GFP construct and a plasmid expressing FLAG-hDaxx. In indirect immunofluorescence analyses with the monoclonal anti-FLAG antibody in order to detect cotransfected hDaxx, we observed that cells expressing both proteins had enlarged ND10 domains in which pp71-GFP and hDaxx perfectly colocalized (Fig. 6Aa to c). This recruitment of pp71-GFP by FLAG-hDaxx was even more obvious in HeLa cells: here, pp71 alone showed a mainly nuclear diffuse staining (Fig. 6Ba to c). The presence of FLAG-hDaxx, however, resulted in a redistribution of pp71-GFP to nuclear dots (Fig. 6Bd to f). This effect was highly reproducible in several independent experiments.
FIG. 6.
Recruitment of pp71 after expression of FLAG-hDaxx. HFF cells (A) and HeLa cells (B) grown on coverslips were transfected with eukaryotic expression vectors encoding pp71-GFP, FLAG-hDaxx, and FLAG-Sp100, as well as the HCMV proteins ppUL69 and IE2, as indicated. Thereafter, indirect immunofluorescence analyses were carried out. pp71 was visible through its GFP moiety (panels Aa, d, k, and n; panels Ba and d). FLAG-hDaxx and FLAG-Sp100 were detected with the anti-FLAG monoclonal antibody (panels Ab and e; panel Be). ppUL69 was stained by using the polyclonal anti-UL69 antiserum (panels Ag and l); IE2 was detected with monoclonal antibody MAb810 (panel Ao). Staining for endogenous hDaxx was performed with a polyclonal anti-hDaxx antiserum (panel Bb). Thereafter, TRITC-conjugated anti-mouse secondary antibodies, as well as FITC-conjugated anti-rabbit secondary antibodies, were employed.
In order to exclude that overexpression of ND10 proteins in general resulted in pp71 recruitment into nuclear dots, we transfected pp71-GFP, together with an expression vector for FLAG-Sp100 into HFF cells, and again analyzed the localization of the proteins via indirect immunofluorescence. As can be seen in Fig. 6A, overexpression of Sp100 revealed large ND10-like domains as was also observed after overexpression of hDaxx (see panels b and e); however, pp71-GFP was not recruited into these structures by Sp100 (see panels d to f). In addition, further control experiments were performed to confirm that recruitment of pp71 was a specific effect mediated by hDaxx. First, we could exclude that overexpression of hDaxx affected the subnuclear localization of the HCMV tegument protein ppUL69 (Fig. 6Ag to i); furthermore, the presence of both ppUL69 and pp71-GFP within one cell did not alter the distribution of each protein (Fig. 6Aj to m). Second, we investigated whether the HCMV transactivator protein IE2-p86, which is known to be associated with ND10 domains, would be able to recruit pp71-GFP (1, 39, 71). Both pp71 and IE2-p86 colocalized in nuclear dots but did not show an enhanced targeting of ND10 domains (Fig. 6An to p). Taken together, pp71-GFP is specifically recruited to ND10 domains when hDaxx is coexpressed in HFF cells as well as in HeLa cells.
The pp71 virion component is able to target nuclear domains immediately after HCMV infection of fibroblast cells.
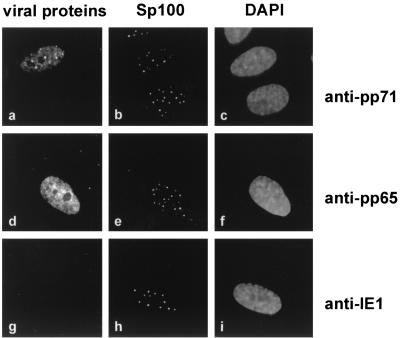
Having shown that pp71 localized to ND10 domains after transient transfection, we wanted to determine whether the pp71 tegument component could also be found in nuclear dots after HCMV infection. In order to prevent viral de novo protein synthesis, HFF cells were cultured in the presence of cycloheximide 30 min prior to infection and extending for 7 h after infection. Thereafter, immunofluorescence analyses were carried out with monoclonal antibodies against pp71, pp65 and IE1 in combination with the anti-Sp26 antiserum against Sp100. In the absence of viral protein expression, pp71 could be detected within the nucleus of infected cells as reported elsewhere (30). Moreover, a clear colocalization with ND10 domains was found in a representative number of nuclei (Fig. 7a and b). The tegument protein pp65 was distributed in a nuclear diffuse pattern that is consistent with findings by others but did not form dot-like structures (Fig. 7d and e) (28). As determined by staining against the IE1 protein, IE gene expression was prevented under these conditions, indicating an effective inhibition of protein synthesis (Fig. 7g and h). Therefore, the pp71 virion component is not only delivered to the nucleus of infected cells but also deposited at the ND10 domains.
FIG. 7.
Subcellular localization of pp71 in HCMV infected cells. HFF cells were treated with 100 μg of cycloheximide/ml 30 min prior to infection (0.5 PFU/cell) and extending for 7 h after infection. Indirect immunofluorescence analyses were carried out with monoclonal antibodies directed against pp71 (a), pp65 (d) and IE1 (g). ND10 domains were visualized by staining the Sp100 protein (b, e, and h). DAPI staining of the respective cell nuclei is shown in panels c, f, and i.
Deletion of putative DIDs within pp71 results in a loss of ND10 localization.
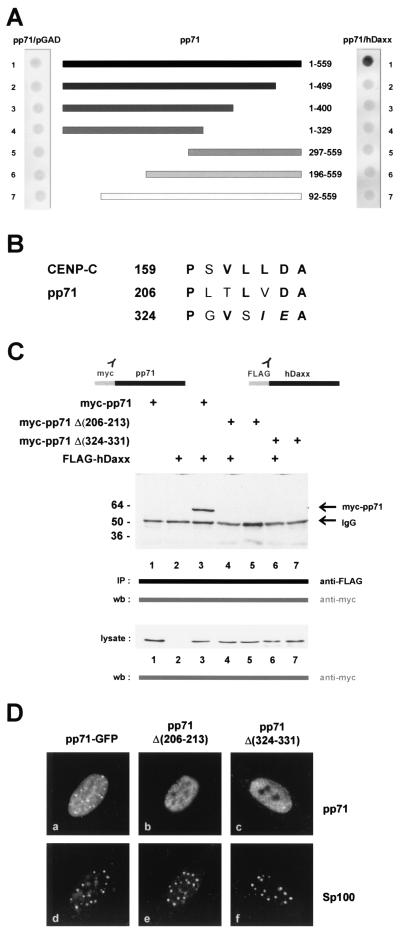
Next, we were interested in determining which part of pp71 was necessary for binding to hDaxx. A series of pp71 deletion mutants in the context of the GAL4 DNA-binding domain was constructed and tested for hDaxx binding by using the yeast two-hybrid system (Fig. 8A). However, we observed that deletions of both the ultimate N- or C-terminal amino acids completely abolished hDaxx interaction. Since it was not possible to narrow down a putative hDaxx binding site within pp71 by 5′ or 3′ deletion analyses, we tried to identify possible internal interaction domains by computer sequence analysis. Since a conserved hDaxx interaction domain (DID) mediating interaction of ETS transcription factors with hDaxx had been described in a previous publication, we performed a search for homologous sequences within pp71 (41). Hereby, we identified two motifs, involving amino acids 206 to 213 amino acids and 324 to 331, which exhibited significant similarity to the DID within CENP-C (Fig. 8B) (41). In order to investigate the potential importance of these amino acids, we internally deleted the two motifs, resulting in mutants pp71Δ(206-213) and pp71Δ(324-331), respectively. These mutants were subsequently analyzed for interaction with hDaxx by either the yeast two-hybrid system (data not shown) or coimmunoprecipitation experiments (Fig. 8C). We observed that both mutants no longer interacted with hDaxx after precipitation from transfected 293 cells, although the proteins were expressed in sufficient amounts and at equal levels, as determined by Western blot analysis (Fig. 8C, lysate). In order to test whether the loss of hDaxx interaction would affect the subnuclear localization of the pp71 mutants, HFF cells were transfected with the respective expression constructs; these analyses were followed by indirect immunofluorescence experiments. Interestingly, both mutants exhibited a microspeckled nuclear pattern, which was clearly distinct from ND10 domains (Fig. 8D, compare panels a and d with panels b and e and panels c and f). Furthermore, overexpression of FLAG-hDaxx did not recruit these mutants into nuclear dots (data not shown). We concluded from these experiments that the deletion of DIDs within pp71 not only affected binding to hDaxx but also abolished the targeting to ND10 domains.
FIG. 8.
Analyses of pp71 deletion mutants. (A) Mapping of DIDs within pp71. Yeast cells were transformed with two separate vectors, one of which encoded full-length pp71 (lane 1) or amino- and carboxy-terminal deletion mutants fused to the GAL4 DNA-binding domain (lanes 2 to 7); the amino acids contained in the respective deletion mutants are indicated. The second plasmid encoded either the empty GAL4 activation domain (lefthand side) or the hDaxx fusion (righthand side). Yeast colonies were selected for the presence of both plasmids on dropout medium lacking tryptophane and leucine and subsequently analyzed for the expression of β-galactosidase by filter lift assays. (B) Specific amino acid sequence homologies between theDIDs of CENP-C and HCMV pp71 (41). Within pp71, internal deletion mutagenesis was performed, resulting in mutants pp71Δ(206-213) and pp71Δ(324-331), respectively. (C) Loss of interaction between pp71 deletion mutants and hDaxx as determined by coimmunoprecipitation experiments. 293 cells were transfected with eukaryotic expression vectors encoding myc-pp71, the internal deletion mutants myc-pp71Δ(206-213) and myc-pp71Δ(324-331), and FLAG-hDaxx as indicated and prepared for immunoprecipitation as described in Materials and Methods. Immunoprecipitation was performed with the monoclonal anti-FLAG antibody. Precipitates were washed three times and separated by SDS-10% PAGE. Thereafter, coprecipitated pp71 proteins were detected in Western blot analysis with the anti-myc monoclonal antibody. Lanes: 1, transfection with plasmid myc-pp71 alone; 2, transfection with vector FLAG-hDaxx alone; 3, transfection with a combination of vectors encoding myc-pp71 and FLAG-hDaxx; 4, transfection with plasmid myc-pp71Δ(206-213), together with the FLAG-hDaxx vector; 5, transfection of plasmid myc-pp71Δ(206-213) alone; 6, combination of plasmids myc-pp71Δ(324-331) and FLAG-hDaxx; 7, transfection of vector myc-pp71Δ(324-331) alone. Prior to immunoprecipitation, an aliquot of each sample was analyzed for expression of pp71 and the respective mutants, as determined by Western blot with the anti-myc monoclonal antibody (lysate; lower part of panel C). Molecular masses are indicated in kilodaltons. Abbreviations: IP, immunoprecipitation; wb, Western blot; IgG, immunoglobulin G. (D) Subcellular localization of the pp71 mutants in HFF cells. HFF cells grown on coverslips were transfected with an expression vector encoding pp71-GFP as control (panel a) and the mutants pp71Δ(206-213) and pp71Δ(324-331) (panels b and c, respectively). ND10 domains were visualized employing the polyclonal Sp26 serum detecting Sp100, followed by incubation with a TRITC-conjugated anti-rabbit secondary antibody (panels d, e, and f).
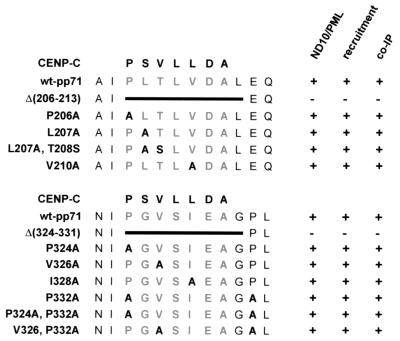
In order to more specifically analyze these two potential DIDs within pp71, we performed alanine exchanges of amino acids in the respective domains as listed in Fig. 9. The resulting point mutants were subsequently analyzed for ND10 association and hDaxx-mediated recruitment into nuclear dots via indirect immunofluorescence analyses.We did not observe any difference in neither ND10 targeting nor recruitment of the mutants in comparison to wild-type pp71. This correlated with the finding that each individual mutant could be coprecipitated with hDaxx after transient expression in 293 cells (data are summarized in Fig. 9). Thus, although two domains with homology to the DID of CENP-C exist within pp71, we were not able to precisely define amino acids within these domains which are essential for hDaxx binding.
FIG. 9.
Analyses of pp71 point mutants. Within the two potential DIDs of pp71, several amino acid residues were substituted by alanine as indicated in the left part of the figure. These mutants were expressed as GFP fusion proteins and were tested for ND10 localization via indirect immunofluorescence staining of the PML protein (ND10/PML); subsequently, recruitment of the inividual mutants was investigated after coexpression of FLAG-hDaxx (recruitment). Additionally, all mutants were expressed as myc-fusions and tested for hDaxx binding in coimmunoprecipitation experiments (co-IP). Positive or negative results in the individual experiments are indicated by “+” or “−.” Wild-type pp71 (wt-pp71) and the internal deletion mutants [Δ(206-213) and Δ(324-331)] were tested in parallel.
hDaxx enhances pp71-mediated transactivation of the HCMV MIEP.
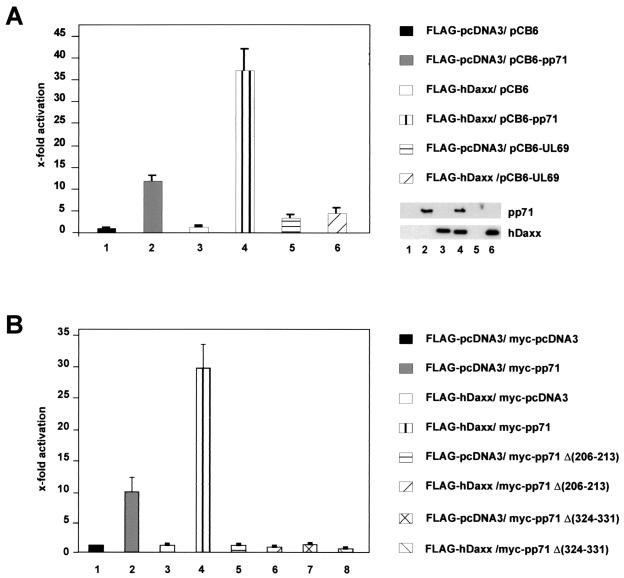
The tegument protein pp71 had been described to be a potent activator of a variety of viral as well as cellular promoters (17, 34, 42, 74). In particular, recent results obtained with a UL82 deletion virus suggested that activation of the HCMV MIEP by pp71 is required for efficient viral replication (15). Therefore, we wondered whether the interaction with hDaxx would influence pp71-mediated transactivation. For this, HCMV permissive U373MG cells were transiently transfected with a reporter construct containing the luciferase gene under the control of the HCMV MIEP and expression vectors for FLAG-hDaxx and pp71 (termed pCB6-pp71) (30) either alone or in combination. As indicated by Fig. 10A, hDaxx had no influence on the reporter gene when expressed alone (Fig. 10A, bar 3). When pCB6-pp71 was used for transfection, we observed an approximately 12-fold activation of the MIEP which is consistent with previously published results (Fig. 10A, bar 2) (9, 42). Interestingly, when pp71 and hDaxx were coexpressed, this activation was further elevated, reaching levels of over 30-fold (Fig, 10A, bar 4). By Western blot analysis, we excluded that the enhanced activation was due to an increased amount of pp71 protein in the presence of hDaxx (Fig. 10A, insert). This suggested that the recruitment of pp71 to ND10 domains by hDaxx correlates with enhanced transactivation by pp71. Again, in order to determine the specificity of the observed effects, we expressed hDaxx together with the HCMV tegument protein ppUL69, which had also been reported to be able to transactivate the MIEP (9, 74). However, coexpression of hDaxx had no significant influence on the transactivation potential of ppUL69 (Fig. 10A, bars 5 and 6).
FIG. 10.
Luciferase analyses after cotransfection of a reporter construct carrying the HCMV MIEP with expression vectors for wild-type pp71 or the pp71 mutants pp71Δ(206-213) and pp71Δ(324-331), FLAG-hDaxx and ppUL69 as indicated. Each experiment was performed in triplicate and repeated at least three times. The fold activation was calculated relative to the basal activity of the reporter construct after cotransfection of the empty expression vectors pCB6 or myc-pcDNA3 and FLAG-pcDNA3. Western blot analyses reveal that similiar amounts of pp71 and hDaxx were expressed (insert). (A) Lanes: 1, cotransfection was performed with the empty expression vectors pCB6 and FLAG-pcDNA3; 2, cotransfection was performed with a construct expressing pp71 (pCB6-pp71); 3, cotransfection was performed with the FLAG-hDaxx plasmid; 4, cotransfection was performed with vectors encoding pp71 and FLAG-hDaxx; 5, cotransfection was performed with a plasmid expressing ppUL69 (pCB6-UL69); 6, cotransfection was performed with plasmids encoding ppUL69 and FLAG-hDaxx. (B) Lanes: 1, cotransfection was performed with the empty expression vectors FLAG-pcDNA3 and myc-pcDNA3; 2, cotransfection was performed with a construct expressing myc-pp71; 3, cotransfection was performed with the FLAG-hDaxx plasmid; 4, cotransfection was performed with vectors encoding myc-pp71 and FLAG-hDaxx; 5, cotransfection was performed with a plasmid expressing the mutant myc-pp71Δ(206-213); 6, cotransfection was performed with vectors encoding myc-pp71Δ(206-213) and FLAG-hDaxx; 7, cotransfection was performed with a plasmid expressing the mutant myc-pp71Δ(324-331); 8, cotransfection was performed with plasmids encoding myc-pp71Δ(324-331) and FLAG-hDaxx.
We performed additional transfection experiments with the internal pp71 deletion mutants pp71Δ(206-213) and pp71Δ(324-331). As shown in Fig. 10B, both mutants were not able to transactivate the HCMV MIEP above background, indicating that their transactivation capacity was completely lost (Fig. 10B, bars 5 and 7). Furthermore, a simultaneous expression of hDaxx did not result in enhanced transactivation as observed for wild-type pp71 (Fig. 10B, bars 6 and 8). Thus, the loss of hDaxx binding and ND10 localization severely affected the transactivating potential of the pp71 internal deletion mutants. In contrast, all pp71 point mutants within the putative DIDs which were able to bind to hDaxx were still able to transactivate and exhibited an enhanced activity after coexpression of hDaxx (data not shown). In summary, these experiments suggested that the recruitment of pp71 by hDaxx strongly contributed to pp71-mediated transactivation.
DISCUSSION
The tegument protein pp71 is encoded by the open reading frame UL82 of HCMV. As a part of the virus particle, pp71 is imported into the nucleus directly after infection, where it is thought to activate the HCMV MIEP in synergism with other tegument proteins (9, 30, 74). Since recombinant HCMV lacking the UL82 open reading frame exhibits a severe growth defect at a low multiplicity of infection, pp71 appears to be essential for efficient lytic replication (14). Furthermore, it was observed that a virus lacking pp71 failed to activate IE gene expression, demonstrating that the pp71 virion component is required for transactivation of the HCMV MIEP (15). The mechanism by which pp71 activates gene expression, however, is not yet understood in detail. The VP16 protein, the functional analogue in HSV, has been shown to require cellular cofactors for transactivation (57). By yeast two-hybrid screening, we were therefore looking for cellular interaction partners of pp71, which might contribute to the transactivating functions of this protein. We isolated here the hDaxx protein as a strong binding partner of pp71.
The hDaxx protein is very conserved in higher eukaryotes and is widely expressed in adult tissues (37, 75). Furthermore, targeted disruption of the hDaxx gene locus in mouse resulted in early embryonic lethality, defining also an important developmental role for this protein (49). Currently, the biochemical functions of hDaxx are not fully understood but seem to be linked to the cellular localization of the protein. HDaxx was originally identified as an adapter molecule bridging the Fas death domain with the cytoplasmic apoptosis signal-regulating kinase ASK-1, resulting in activation of JNK and p38 mitogen-activated protein kinase and thereby Fas-induced apoptosis (16, 38, 75). Other studies, however, have suggested that hDaxx indeed is dispensable for Fas-mediated cell death (49, 68). Therefore, the role of hDaxx in either inducing or preventing apoptosis remains a matter of debate.
Apart from a possible role in regulation of signal transduction in the cytoplasm, hDaxx has also been reported to act in the nucleus of a variety of different cell types. It is able to bind to the transcription factors Ets-1 and Pax-3, thereby exerting a repressory effect on respective target genes (33, 41). Furthermore, it was shown that the carboxy terminus of hDaxx strongly repressed transcription, which possibly is due to interaction between hDaxx and histone deacetylases (40, 67). It has been shown that the nuclear localization of hDaxx changed during the cell cycle, since it associates with the centromer protein CENP-C during interphase (54). In addition, hDaxx has been shown to be a constituent of the nuclear domain ND10 as a consequence of interaction with the PML protein (35, 76).
Since hDaxx had been shown to interact with a variety of cellular proteins, we first wanted to investigate whether its binding to pp71 was specific. For this, we employed the yeast two-hybrid system by which we were able to show that hDaxx specifically interacted with pp71, since it did not bind to other HCMV tegument proteins such as pUL26 and ppUL69. Similarly, an interaction between pp71 and other ND10 proteins, such as PML and Sp100, was not detected. These findings were additionally strengthened by coimmunoprecipitation experiments which confirmed the interaction between pp71 and hDaxx but again could exclude binding between pp71 and the ND10 protein Sp100. Moreover, no affinity of hDaxx toward the tegument protein ppUL69 or the HCMV regulatory factor pUL84 was observed. Taken together, we provide evidence that the interaction between pp71 and hDaxx indeed is specific.
Therefore, we wanted to closer define the pp71 binding site within the hDaxx protein. By using the yeast two-hybrid system, we identified a central domain containing a cluster of acidic amino acids within hDaxx as required for pp71 binding. Immunoprecipitation experiments confirmed this central domain as a strong hDaxx binding site. Additionally, we observed that the amino terminus of hDaxx was able to coprecipitate pp71 after expression in mammalian cells. The negative result with this region in the yeast two-hybrid system could possibly be explained by the fact that this interaction was rather weak and might therefore not be detected in yeast or might be due to instability of this mutant in yeast cells. Alternatively, the binding could require posttranslational modification which does not take place in yeast cells. Consistent with the latter, the N-terminal region of hDaxx contains putative phosphorylation sites which could be required for efficient pp71 binding (41). Thus far, all published hDaxx-binding factors had turned out to bind via the carboxy terminus of hDaxx (33, 40, 41, 54, 75). Interestingly, this region was completely dispensable for interaction with pp71 both in yeast and in mammalian cells, thus further confirming the specificity of the interaction. Consistent with this, we could reconfirm that the ND10 protein PML, which was used as a positive control in the yeast experiments, bound via the carboxy terminus of hDaxx, a finding which suggests that both pp71 and PML might bind to hDaxx simultaneously via distinct domains.
The interaction between pp71 and a ND10 component gave rise to the question whether pp71 itself might be present in ND10 domains. In order to elucidate this, we performed transient-transfection experiments in HFF cells with a plasmid expressing pp71 in fusion with GFP. Immunofluorescence analyses revealed that the pp71-GFP protein colocalized with PML, Sp100, and hDaxx in nuclear speckles, indicating that pp71 was indeed able to target ND10 domains. In order to exclude artifacts arising from the fusion with GFP itself, we additionally analyzed the localization of an N-terminal fusion of pp71 to YFP and came to the same results. Since fusions at both the amino terminus and the carboxy terminus of pp71 had no influence on ND10 localization, we concluded that pp71 itself carried the information for ND10 targeting. In summary, our data suggest that, apart from already-known ND10-associated HCMV regulatory proteins such as IE1-p72, IE2-p86, and UL112-113, the tegument protein pp71 is also able to target ND10 domains independent of other viral proteins (1, 3, 39, 71).
Moreover, coexpression of hDaxx drastically altered the distributon of pp71-GFP, since we observed a strong recruitment of pp71 to ND10 domains. This recruitment was not a general effect after overexpression of ND10-associated factors, since a construct expressing FLAG-Sp100 was not able to recruit pp71, although FLAG-Sp100 localized to enlarged nuclear dots comparable to the speckles observed after overexpression of FLAG-hDaxx (2, 29, 35, 40, 67). The specificity of hDaxx-mediated recruitment of pp71 was further strengthened by the observation that the intracellular localization of another HCMV regulatory protein, the tegument protein ppUL69, was not affected after coexpression of hDaxx. Furthermore, overexpression of the ND10-associated viral regulatory protein IE2-p86 did not alter the subcellular distribution of pp71.
Finally, in an attempt to define the hDaxx binding domain within pp71, we observed that two internal deletion mutants of pp71 lacking potential DIDs as described for CENP-C and ETS-factors were no longer able to bind hDaxx (41). This correlated with a failure to accumulate in ND10 even after overexpression of hDaxx. However, when site-directed mutagenesis experiments were performed we were not able to identify individual amino acid positions within the putative DIDs with a critical function for binding. This may indicate that an alteration of the three-dimensional protein structure after internal deletion of the putative DIDs is responsible for the observed loss of interaction. The loss of interaction after deletion of N- and C-terminal sequences as observed in the yeast two-hybrid system also supports the view that the structure of pp71 is easily affected by deletions. Thus, further experiments will be required to unequivocally identify the domain within pp71 that is directly involved in contacting hDaxx.
In summary, we provide evidence that hDaxx is able to specifically recruit pp71 to ND10 domains. Since hDaxx itself localizes within ND10 as a consequence of interaction with the PML protein (35, 40), hDaxx might serve as an adapter polypeptide for the ND10 localization of this viral protein. This would be consistent with the following findings of other groups. First, pp71 has previously been observed in a punctate nuclear pattern in transient-expression experiments which might correlate to the ND10 localization observed in this study (30). Second, HCMV genomes are targeted to ND10 domains immediately after infection, and it was shown that only viral genomes adjacent to ND10 give rise to IE mRNAs, suggesting that ND10 represent the site of active viral transcription immediately after infection (36). Since pp71 was previously identified as a transactivator of viral IE gene expression, a colocalization with viral genomes in the vicinity of ND10 might be required for pp71 in order to directly affect gene expression during infection.
This hypothesis was investigated via indirect immunofluorescence experiments with HCMV-infected cells. In order to specifically detect the pp71 protein as imported by viral particles, cells were treated with cycloheximide prior to infection. This treatment efficiently abolished de novo protein synthesis since no IE gene expression was detectable in the infected culture. Consistent with previous findings, we observed the translocation of incoming pp71 protein to the nucleus (30). Moreover, pp71 appeared in nuclear speckles which perfectly overlapped with the ND10 factor Sp100, indicating that pp71 targeted these nuclear domains also in the context of viral infection. As a control, the abundant tegument protein pp65 was employed, which gave a nuclear diffuse signal under these conditions as reported by others but did not colocalize with ND10 domains (28). In summary, pp71 was found to target ND10 after transient expression, as well as after viral infection.
Finally, we addressed the question whether hDaxx-mediated recruitment to ND10 could influence transactivation by pp71. For this, we performed luciferase reporter experiments in HCMV-permissive U373MG cells. We observed a synergistic transactivation of the HCMV MIEP when pp71 and hDaxx were coexpressed, whereas hDaxx alone had no effect on the basal activity of the reporter construct. Therefore, we concluded that pp71-mediated transactivation could be strongly enhanced if pp71 was tethered to ND10 domains by hDaxx. However, an indirect effect of hDaxx on pp71-mediated transactivation which is independent from ND10 localization cannot be excluded entirely at present. The synergistic activation measured after coexpression of pp71 and hDaxx is surprising in the light of published data describing hDaxx as a transcriptional repressor (33, 41, 67). Our finding, however, is consistent with findings by Li et al., who showed that PML is able to inhibit hDaxx-mediated repression, which in turn correlates with the recruitment of hDaxx to ND10 domains (40). In conclusion, the ND10 domain might regulate transcription at two different levels. First, a number of both cellular and viral transcriptional coactivators are able to target ND10 domains (for a review, see reference 59). Second, ND10 domains may sequester transcriptional repressors such as hDaxx, thereby preventing them to exert their inhibitory function on respective target genes. HCMV additionally takes advantage of the latter, since the deposition of hDaxx at ND10 allows for localization of pp71 at these domains, which in turn is required for efficient activation of IE gene expression.
Acknowledgments
We thank Donatella de Gaspero-Hoops and Regina Kupfer for excellent technical assistance. We also thank B. Britt, G. Maul, S. Lang, T. Shenk, and T. Sternsdorf for reagents and B. Fleckenstein for continuous support.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB473) and the Bundesministerium für Forschung und Technologie (IZKF Erlangen). In addition, H.S. was supported by an EMBO short-term fellowship award.
REFERENCES
- 1.Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18:4899-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. [DOI] [PubMed] [Google Scholar]
- 3.Ahn, J. H., W. J. Jang, and G. S. Hayward. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alford, C. A., and W. J. Britt. 1990. Cytomegalovirus, p. 1981-2010. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Raven Press, Ltd., New York, N.Y.
- 5.Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. [DOI] [PubMed] [Google Scholar]
- 6.Angulo, A., C. Suto, R. A. Heyman, and P. Ghazal. 1996. Characterization of the sequences of the human cytomegalovirus enhancer that mediate differential regulation by natural and synthetic retinoids. Mol. Endocrinol. 10:781-793. [DOI] [PubMed] [Google Scholar]
- 7.Arlt, H., D. Lang, S. Gebert, and T. Stamminger. 1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol. 68:4117-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 9.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannister, A. J., and T. Kouzarides. 1996. The CBP coactivator is a histone acetyltransferase. Nature 384:641-643. [DOI] [PubMed] [Google Scholar]
- 12.Boshart, M., F. Weber, G. Jahn, K. Dorsch-Hasler, B. Fleckenstein, and W. Schaffner. 1985. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41:521-530. [DOI] [PubMed] [Google Scholar]
- 13.Breeden, L., and K. Nasmyth. 1985. Regulation of the yeast HO gene. Cold Spring Harbor Symp. Quant. Biol. 50:643-650. [DOI] [PubMed] [Google Scholar]
- 14.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860-1863. [DOI] [PubMed] [Google Scholar]
- 17.Chau, N. H., C. D. Vanson, and J. A. Kerry. 1999. Transcriptional regulation of the human cytomegalovirus US11 early gene. J. Virol. 73:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien, C. T., P. L. Bartel, R. Sternglanz, and S. Fields. 1991. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc. Natl. Acad. Sci. USA 88:9578-9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMarchi, J. M. 1981. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology 114:23-38. [DOI] [PubMed] [Google Scholar]
- 20.Durfee, T., K. Becherer, P. L. Chen, S. H. Yeh, Y. Yang, A. E. Kilburn, W. H. Lee, and S. J. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7:555-569. [DOI] [PubMed] [Google Scholar]
- 21.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 22.Gebert, S., S. Schmolke, G. Sorg, S. Floss, B. Plachter, and T. Stamminger. 1997. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J. Virol. 71:7048-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazal, P., H. Lubon, C. Reynolds-Kohler, L. Hennighausen, and J. A. Nelson. 1990. Interactions between cellular regulatory proteins and a unique sequence region in the human cytomegalovirus major immediate-early promoter. Virology 174:18-25. [DOI] [PubMed] [Google Scholar]
- 24.Gibson, W. 1981. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology 111:516-537. [DOI] [PubMed] [Google Scholar]
- 25.Gibson, W. 1983. Protein counterparts of human and simian cytomegaloviruses. Virology 128:391-406. [DOI] [PubMed] [Google Scholar]
- 26.Gietz, D. S., R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greaves, R. F., and E. S. Mocarski. 1998. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J. Virol. 72:366-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grefte, J. M., B. T. van der Gun, S. Schmolke, M. van der Giessen, W. J. van Son, B. Plachter, G. Jahn, and T. H. The. 1992. The lower matrix protein pp65. is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J. Gen. Virol. 73:2923-2932. [DOI] [PubMed] [Google Scholar]
- 29.Guo, A., P. Salomoni, J. Luo, A. Shih, S. Zhong, W. Gu, and P. P. Paolo. 2000. The function of PML in p53-dependent apoptosis. Nat. Cell Biol. 2:730-736. [DOI] [PubMed] [Google Scholar]
- 30.Hensel, G. M., H. H. Meyer, I. Buchmann, D. Pommerehne, S. Schmolke, B. Plachter, K. Radsak, and H. F. Kern. 1996. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. J. Gen. Virol. 77:3087-3097. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann, H., S. Floss, and T. Stamminger. 2000. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 74:2510-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollenbach, A. D., J. E. Sublett, C. J. McPherson, and G. Grosveld. 1999. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 18:3702-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homer, E. G., A. Rinaldi, M. J. Nicholl, and C. M. Preston. 1999. Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71. J. Virol. 73:8512-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 138:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiriakidou, M., D. A. Driscoll, J. M. Lopez-Guisa, and J. F. Strauss III. 1997. Cloning and expression of primate Daxx cDNAs and mapping of the human gene to chromosome 6p21.3 in the MHC region. DNA Cell Biol. 16:1289-1298. [DOI] [PubMed] [Google Scholar]
- 38.Ko, Y. G., Y. S. Kang, H. Park, W. Seol, J. Kim, T. Kim, H. S. Park, E. J. Choi, and S. Kim. 2001. Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm. J. Biol. Chem. 276:39103-39106. [DOI] [PubMed] [Google Scholar]
- 39.Korioth, F., G. G. Maul, B. Plachter, T. Stamminger, and J. Frey. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229:155-158. [DOI] [PubMed] [Google Scholar]
- 40.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, R., H. Pei, D. K. Watson, and T. S. Papas. 2000. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene 19:745-753. [DOI] [PubMed] [Google Scholar]
- 42.Liu, B., and M. F. Stinski. 1992. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J. Virol. 66:4434-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubon, H., P. Ghazal, L. Hennighausen, C. Reynolds-Kohler, C. Lockshin, and J. Nelson. 1989. Cell-specific activity of the modulator region in the human cytomegalovirus major immediate-early gene. Mol. Cell. Biol. 9:1342-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maul, G. G., D. Negorev, P. Bell, and A. M. Ishov. 2000. Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129:278-287. [DOI] [PubMed] [Google Scholar]
- 46.Mazeron, M. C., G. Jahn, and B. Plachter. 1992. Monoclonal antibody E-13 (McAb 810) to human cytomegalovirus recognizes an antigen encoded by exon 2 of the major immediate-early gene. J. Gen. Virol. 73:2699-2703. [DOI] [PubMed] [Google Scholar]
- 47.McDonough, S. H., and D. H. Spector. 1983. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology 125:31-46. [DOI] [PubMed] [Google Scholar]
- 48.Meier, J. L., and M. F. Stinski. 1996. Regulation of human cytomegalovirus immediate-early gene expression. Intervirology 39:331-342. [DOI] [PubMed] [Google Scholar]
- 49.Michaelson, J. S., D. Bader, F. Kuo, C. Kozak, and P. Leder. 1999. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev. 13:1918-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe and P. M. Howley (ed.), Virology. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 51.Mocarski, E. S., G. W. Kemble, J. M. Lyle, and R. F. Greaves. 1996. A deletion mutant in the human cytomegalovirus gene encoding IE1(491aa) is replication defective due to a failure in autoregulation. Proc. Natl. Acad. Sci. USA 93:11321-11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nowak, B., C. Sullivan, P. Sarnow, R. Thomas, F. Bricout, J. C. Nicolas, B. Fleckenstein, and A. J. Levine. 1984. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology 132:325-338. [DOI] [PubMed] [Google Scholar]
- 53.Pizzorno, M. C., M. A. Mullen, Y. N. Chang, and G. S. Hayward. 1991. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J. Virol. 65:3839-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pluta, A. F., W. C. Earnshaw, and I. G. Goldberg. 1998. Interphase-specific association of intrinsic centromere protein CENP-C with HDaxx, a death domain-binding protein implicated in Fas-mediated cell death. J. Cell Sci. 111(Pt. 14):2029-2041. [DOI] [PubMed] [Google Scholar]
- 55.Puchtler, E., and T. Stamminger. 1991. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J. Virol. 65:6301-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roby, C., and W. Gibson. 1986. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J. Virol. 59:714-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Virology. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 58.Ruger, B., S. Klages, B. Walla, J. Albrecht, B. Fleckenstein, P. Tomlinson, and B. Barrell. 1987. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 61:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seeler, J. S., and A. Dejean. 1999. The PML nuclear bodies: actors or extras? Curr. Opin. Genet. Dev. 9:362-367. [DOI] [PubMed] [Google Scholar]
- 60.Shelbourn, S. L., S. K. Kothari, J. G. Sissons, and J. H. Sinclair. 1989. Repression of human cytomegalovirus gene expression associated with a novel immediate early regulatory region binding factor. Nucleic Acids Res. 17:9165-9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spaete, R. R., R. C. Gehrz, and M. P. Landini. 1994. Human cytomegalovirus structural proteins. J. Gen. Virol. 75(Pt. 12):3287-3308. [DOI] [PubMed] [Google Scholar]
- 62.Spaete, R. R., and E. S. Mocarski. 1985. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J. Virol. 56:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stamminger, T., H. Fickenscher, and B. Fleckenstein. 1990. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J. Gen. Virol. 71:105-113. [DOI] [PubMed] [Google Scholar]
- 64.Stamminger, T., E. Puchtler, and B. Fleckenstein. 1991. Discordant expression of the immediate-early 1 and 2 gene regions of human cytomegalovirus at early times after infection involves posttranscriptional processing events. J. Virol. 65:2273-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenberg, R. M., A. S. Depto, J. Fortney, and J. A. Nelson. 1989. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J. Virol. 63:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stinski, M. F., and T. J. Roehr. 1985. Activation of the major immediate-early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J. Virol. 55:431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torii, S., D. A. Egan, R. A. Evans, and J. C. Reed. 1999. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs). EMBO J. 18:6037-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villunger, A., D. C. Huang, N. Holler, J. Tschopp, and A. Strasser. 2000. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involve DAXX, RIP, or RAIDD. J. Immunol. 165:1337-1343. [DOI] [PubMed] [Google Scholar]
- 69.Wathen, M. W., and M. F. Stinski. 1982. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J. Virol. 41:462-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wathen, M. W., D. R. Thomsen, and M. F. Stinski. 1981. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J. Virol. 38:446-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkinson, G. W., C. Kelly, J. H. Sinclair, and C. Rickards. 1998. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J. Gen. Virol. 79:1233-1245. [DOI] [PubMed] [Google Scholar]
- 72.Winkler, M., S. T. aus Dem, and T. Stamminger. 2000. Functional interaction between pleiotropic transactivator pUL69 of human cytomegalovirus and the human homolog of yeast chromatin regulatory protein SPT6. J. Virol. 74:8053-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winkler, M., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 68:3943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler, M., S. Schmolke, B. Plachter, and T. Stamminger. 1995. UL69 of HCMV, a homolog of the HSV ICP27, is contained within the tegument of virions and activates the major IE enhancer of HCMV in synergy with the tegument protein pp71. Scand. J. Infect. Dis. Suppl. 99:8-9. [Google Scholar]
- 75.Yang, X., R. Khosravi-Far, H. Y. Chang, and D. Baltimore. 1997. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong, S., P. Salomoni, S. Ronchetti, A. Guo, D. Ruggero, and P. P. Pandolfi. 2000. Promyelocytic leukemia protein (PML) and Daxx participate in a novel nuclear pathway for apoptosis. J. Exp. Med. 191:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]