Abstract
The biogenesis of transfer RNA is a process that requires many different factors. In this study, we describe a genetic screen aimed to identify gene products participating in this process. By screening for mutations lethal in combination with a sup61-T47:2C allele, coding for a mutant form of 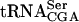 , the nonessential TAN1 gene was identified. We show that the TAN1 gene product is required for formation of the modified nucleoside N4-acetylcytidine (ac4C) in tRNA. In Saccharomyces cerevisiae, ac4C is present at position 12 in tRNAs specific for leucine and serine as well as in 18S ribosomal RNA. Analysis of RNA isolated from a tan1-null mutant revealed that ac4C was absent in tRNA, but not rRNA. Although no tRNA acetyltransferase activity by a GST-Tan1 fusion protein was detected, a gel-shift assay revealed that Tan1p binds tRNA, suggesting a direct role in synthesis of ac4C12. The absence of the TAN1 gene in the sup61-T47:2C mutant caused a decreased level of mature
, the nonessential TAN1 gene was identified. We show that the TAN1 gene product is required for formation of the modified nucleoside N4-acetylcytidine (ac4C) in tRNA. In Saccharomyces cerevisiae, ac4C is present at position 12 in tRNAs specific for leucine and serine as well as in 18S ribosomal RNA. Analysis of RNA isolated from a tan1-null mutant revealed that ac4C was absent in tRNA, but not rRNA. Although no tRNA acetyltransferase activity by a GST-Tan1 fusion protein was detected, a gel-shift assay revealed that Tan1p binds tRNA, suggesting a direct role in synthesis of ac4C12. The absence of the TAN1 gene in the sup61-T47:2C mutant caused a decreased level of mature 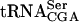 , indicating that ac4C12 and/or Tan1p is important for tRNA stability.
, indicating that ac4C12 and/or Tan1p is important for tRNA stability.
Keywords: N4-acetylcytidine, YGL232w, sup61, tRNA maturation, tRNA modification
INTRODUCTION
Eukaryotic transfer RNA genes are transcribed by RNA polymerase III generating precursor tRNAs that have to undergo a series of processing events to yield mature functional tRNAs (Hopper and Phizicky 2003). During the maturation of tRNA, a variety of different nucleoside modifications occur. Modified nucleosides are found in all phylogenetic domains and also in identical positions of the tRNA, suggesting a conserved function of some tRNA modifications (Björk 1986; Cermakian and Cedergren 1998; Björk et al. 2001). The modified nucleoside N4-acetylcytidine (ac4C) is found in tRNA from organisms within all three domains (Sprinzl et al. 1998). It is present at position 34 in some archaeal and bacterial tRNAs, and in vitro experiments using Escherichia coli  suggested that ac4C34 prevents misreading of AUA isoleucine codons during protein synthesis (Stern and Schulman 1978). In eukaryotes, ac4C is found only at position 12 in a subset of tRNAs. The tRNAs in Saccharomyces cerevisiae containing ac4C12 are the species for leucine and serine (Sprinzl et al. 1998). The modification of the cytidine at position 12 is an early step in the maturation of tRNAs in S. cerevisiae, as ac4C12 is present in intron containing precursor tRNA (Etcheverry et al. 1979). To date, the biological function of ac4C12 has not been elucidated.
suggested that ac4C34 prevents misreading of AUA isoleucine codons during protein synthesis (Stern and Schulman 1978). In eukaryotes, ac4C is found only at position 12 in a subset of tRNAs. The tRNAs in Saccharomyces cerevisiae containing ac4C12 are the species for leucine and serine (Sprinzl et al. 1998). The modification of the cytidine at position 12 is an early step in the maturation of tRNAs in S. cerevisiae, as ac4C12 is present in intron containing precursor tRNA (Etcheverry et al. 1979). To date, the biological function of ac4C12 has not been elucidated.
The sup61+ gene is a single copy and essential gene encoding 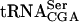 , which is the only serine isoacceptor that decodes UCG codons (Etcheverry et al. 1982). We previously showed (Johansson and Byström 2002) that strains with a mutant sup61 allele have a requirement for the genes encoding the modification enzymes tRNA(m5U54) methyltransferase (Trm2p; Nordlund et al. 2000), pseudouridine(Ψ55) synthase (Pus4p; Becker et al. 1997), tRNA (
, which is the only serine isoacceptor that decodes UCG codons (Etcheverry et al. 1982). We previously showed (Johansson and Byström 2002) that strains with a mutant sup61 allele have a requirement for the genes encoding the modification enzymes tRNA(m5U54) methyltransferase (Trm2p; Nordlund et al. 2000), pseudouridine(Ψ55) synthase (Pus4p; Becker et al. 1997), tRNA (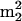 G26) dimethyltransferase (Trm1p; Ellis et al. 1986) and the putative tRNA(Gm18) 2′-O-ribose methyltransferase (Trm3p; Cavaille et al. 1999). The m5U54, Ψ55,
G26) dimethyltransferase (Trm1p; Ellis et al. 1986) and the putative tRNA(Gm18) 2′-O-ribose methyltransferase (Trm3p; Cavaille et al. 1999). The m5U54, Ψ55, 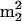 G26, and Gm18 nucleotides are all present in
G26, and Gm18 nucleotides are all present in 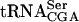 (Etcheverry et al. 1979). Moreover, the LHP1 gene product involved in 3′ end maturation of pre-tRNAs was important for growth of sup61 mutants (Yoo and Wolin 1997; Johansson and Byström 2002). When null alleles of any of these genes were introduced into strains with mutant forms of
(Etcheverry et al. 1979). Moreover, the LHP1 gene product involved in 3′ end maturation of pre-tRNAs was important for growth of sup61 mutants (Yoo and Wolin 1997; Johansson and Byström 2002). When null alleles of any of these genes were introduced into strains with mutant forms of 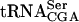 , growth defects were obtained that correlated to a reduced level of
, growth defects were obtained that correlated to a reduced level of 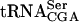 (Johansson and Byström 2002). These data implied that other genes encoding modification/maturation proteins interacting with
(Johansson and Byström 2002). These data implied that other genes encoding modification/maturation proteins interacting with 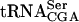 would be essential in the sup61 mutants.
would be essential in the sup61 mutants.
In this study, we report that a sup61-T47:2C mutant requires the open reading frame YGL232w for growth. We show that YGL232w is required for ac4C formation in tRNA and denoted the gene TAN1 for tRNA acetylation. The absence of the Tan1 protein in the sup61-T47:2C strain caused a decreased level of mature  , suggesting that ac4C12 and/or Tan1p is important for tRNA stability.
, suggesting that ac4C12 and/or Tan1p is important for tRNA stability.
RESULTS
A strain with a mutant form of 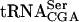 requires the YGL232w open reading frame for growth
requires the YGL232w open reading frame for growth
A yeast strain with the sup61-T47:2C allele (Fig. 1 ▶) requires, to various degrees, any of the LHP1, PUS4, TRM1, and TRM2 genes for growth (Johansson and Byström 2002). This suggested that the sup61-T47:2C allele would cause a requirement for other proteins modifying or interacting with 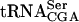 . To identify gene products involved in
. To identify gene products involved in 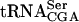 biogenesis, a synthetic lethal screen was performed by using a red/white colony color assay (Kranz and Holm 1990; Bender and Pringle 1991). Briefly, a haploid ura3 ade2 ade3 sup61-T47:2C strain carrying a plasmid with the ADE3, URA3, and sup61+ genes was mutagenized and colonies screened for the inability to lose the plasmid, which is scored as uniformly red (nonsectored) colonies. From ~20,000 colonies, 21 strains with a single recessive mutation were identified that required the plasmid borne sup61+ gene for survival (see Materials and Methods). Complementation analysis of the sup61+-dependent mutants defined 12 complementation groups. We expected to find lhp1, pus4, trm1, and trm2 mutants among the isolates, and one complementation group consisted of two strains with a mutant trm1 allele (data not shown). However, no lhp1, pus4, and trm2 mutants were identified, showing that the screen was not saturated.
biogenesis, a synthetic lethal screen was performed by using a red/white colony color assay (Kranz and Holm 1990; Bender and Pringle 1991). Briefly, a haploid ura3 ade2 ade3 sup61-T47:2C strain carrying a plasmid with the ADE3, URA3, and sup61+ genes was mutagenized and colonies screened for the inability to lose the plasmid, which is scored as uniformly red (nonsectored) colonies. From ~20,000 colonies, 21 strains with a single recessive mutation were identified that required the plasmid borne sup61+ gene for survival (see Materials and Methods). Complementation analysis of the sup61+-dependent mutants defined 12 complementation groups. We expected to find lhp1, pus4, trm1, and trm2 mutants among the isolates, and one complementation group consisted of two strains with a mutant trm1 allele (data not shown). However, no lhp1, pus4, and trm2 mutants were identified, showing that the screen was not saturated.
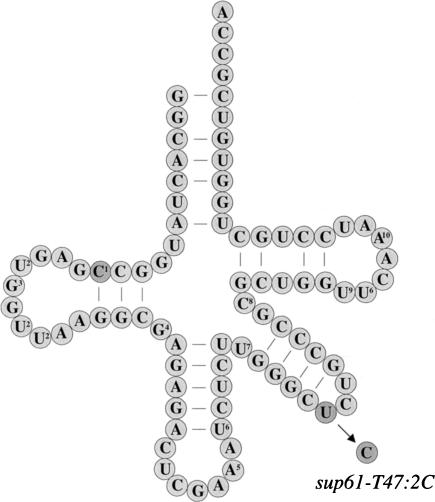
FIGURE 1.
Cloverleaf structure of 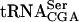 . The position of the sup61-T47:2C mutation and modified nucleosides are indicated where numbers within the circles represent the following modifications: 1, N4-acetylcytidine (ac4C); 2, dihydrouridine (D); 3: 2′-o-methylguanosine (Gm); 4, N2,N2-dimethylguanosine (
. The position of the sup61-T47:2C mutation and modified nucleosides are indicated where numbers within the circles represent the following modifications: 1, N4-acetylcytidine (ac4C); 2, dihydrouridine (D); 3: 2′-o-methylguanosine (Gm); 4, N2,N2-dimethylguanosine (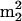 G); 5, N6-isopentenyladenosine (i6A); 6, pseudouridine (Ψ); 7, 2′-O-methyluridine (Um); 8, 5-methylcytidine (m5C); 9, 5-methyluridine (m5U); and 10, Etcheverry et al. (1979) detected a modification at position 57 or 58 that presumably corresponds to 1-methyladenosine (m1A) at position 58.
G); 5, N6-isopentenyladenosine (i6A); 6, pseudouridine (Ψ); 7, 2′-O-methyluridine (Um); 8, 5-methylcytidine (m5C); 9, 5-methyluridine (m5U); and 10, Etcheverry et al. (1979) detected a modification at position 57 or 58 that presumably corresponds to 1-methyladenosine (m1A) at position 58.
In this study, we describe the identification and characterization of the gene mutated in a complementation group consisting of three mutants. By using a yeast genomic library, plasmids that complemented the nonsectoring phenotype were identified. Partial DNA sequencing and subsequent subcloning revealed that the YGL232w open reading frame restored sectoring in the three mutants. Genetic linkage between YGL232w and the original mutation was verified by targeted integration and tetrad analysis (see Materials and Methods). The YGL232w gene, predicted to encode a 33.6-kD protein, is not required for growth in a wild-type background (Giaever et al. 2002). To unambiguously demonstrate the synthetic interaction, the sup61-T47:2C mutation was combined with a ygl232w-null allele. The resulting sup61-T47:2C ygl232wΔ mutant was very slow growing at 25°C and nonviable at 30°C (Fig. 2A ▶; data not shown). Thus, the YGL232w gene (hereafter referred to as TAN1, see below) is required for growth of a strain with a mutant form of 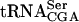 .
.
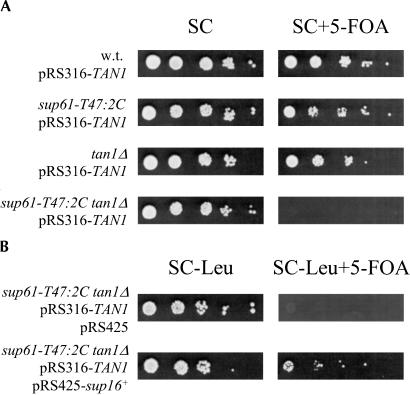
FIGURE 2.
The TAN1 gene is required for growth of a sup61-T47:2C strain. (A) Wild-type (UMY2220), sup61-T47:2C (UMY2256), tan1Δ (UMY2874), and sup61-T47:2C tan1Δ (derived from UMY2951) strains carrying the low copy URA3 plasmid pRS316-TAN1 were grown in synthetic complete medium (SC), serially diluted, spotted onto SC and SC+5-fluoro-orotic acid (5-FOA) plates, and incubated at 30°C for 2 d. Cells containing a URA3 plasmid are unable to grow on 5-FOA–containing media (Boeke et al. 1984). (B) The sup61-T47:2C tan1Δ strain in A transformed with pRS425 or pRS425-sup16+ (high copy LEU2 plasmids) was grown in SC-Leu medium, serially diluted, spotted onto SC-Leu and SC-Leu+5-FOA plates, and incubated for 3 d at 30°C.
Transfer RNA isolated from a tan1-null mutant lacks ac4C
We previously showed that the genes encoding the modification enzymes catalyzing formation of 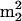 G26, m5U54, and Ψ55 in tRNA were important for growth of the sup61-T47:2C strain (Johansson and Byström 2002). This suggested that the TAN1 gene might encode a tRNA modification enzyme. To investigate this hypothesis, total tRNA from the wild-type strain (UMY2220), containing empty vector, and the tan1-null mutant (UMY2874), carrying either empty vector or plasmid bearing TAN1, was prepared. The tRNA was degraded to nucleosides and subjected to HPLC analysis. The analysis revealed that tRNA from the tan1-null mutant was missing one compound. This compound present in the wild-type and the null mutant carrying the TAN1 plasmid has the retention time and spectrum of N4-acetylcytidine (ac4C; Fig. 3 ▶). To confirm the nature of the compound, synthetic ac4C was added to a wild-type tRNA digest before HPLC analysis. The analysis revealed that the synthetic ac4C comigrated with the compound that is absent in the tan1-null strain (Fig. 3 ▶). The ac4C nucleoside is present at position 12 in tRNAs specific for leucine and serine (Sprinzl et al. 1998), including
G26, m5U54, and Ψ55 in tRNA were important for growth of the sup61-T47:2C strain (Johansson and Byström 2002). This suggested that the TAN1 gene might encode a tRNA modification enzyme. To investigate this hypothesis, total tRNA from the wild-type strain (UMY2220), containing empty vector, and the tan1-null mutant (UMY2874), carrying either empty vector or plasmid bearing TAN1, was prepared. The tRNA was degraded to nucleosides and subjected to HPLC analysis. The analysis revealed that tRNA from the tan1-null mutant was missing one compound. This compound present in the wild-type and the null mutant carrying the TAN1 plasmid has the retention time and spectrum of N4-acetylcytidine (ac4C; Fig. 3 ▶). To confirm the nature of the compound, synthetic ac4C was added to a wild-type tRNA digest before HPLC analysis. The analysis revealed that the synthetic ac4C comigrated with the compound that is absent in the tan1-null strain (Fig. 3 ▶). The ac4C nucleoside is present at position 12 in tRNAs specific for leucine and serine (Sprinzl et al. 1998), including 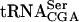 (Etcheverry et al. 1979). In addition to tRNA, 18S rRNA contains ac4C (Thomas et al. 1978). HPLC analysis of nucleosides derived from rRNA revealed that ac4C was present in the tan1-null mutant (data not shown). Thus, the Tan1 protein influenced formation of ac4C in tRNA but not rRNA. Based on these results, the YGL232w open reading frame was denoted TAN1 for tRNA acetylation.
(Etcheverry et al. 1979). In addition to tRNA, 18S rRNA contains ac4C (Thomas et al. 1978). HPLC analysis of nucleosides derived from rRNA revealed that ac4C was present in the tan1-null mutant (data not shown). Thus, the Tan1 protein influenced formation of ac4C in tRNA but not rRNA. Based on these results, the YGL232w open reading frame was denoted TAN1 for tRNA acetylation.
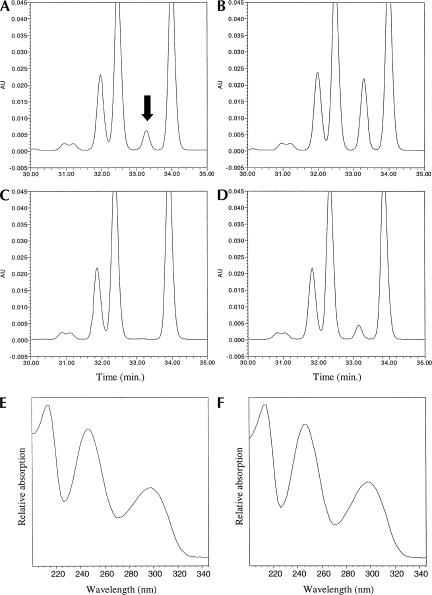
FIGURE 3.
The tan1-null mutant lacks ac4C in tRNA. HPLC analysis of nucleosides derived from total tRNA isolated from wild-type strain (UMY2220) carrying pRS316 (A); wild-type strain (UMY2220) carrying pRS316 with addition of 0.5 nmole synthetic ac4C (B); tan1Δ strain (UMY2874) carrying pRS316 (C); and tan1Δ strain (UMY2874) carrying pRS316-TAN1 (D). Only the portion of the chromatograms between retention times 30 and 35 min are shown. An arrow indicates the peak corresponding to ac4C. (E) UV absorption spectrum of the peak indicated in A. (F) UV absorption spectrum of synthetic ac4C.
The Tan1 protein interacts with tRNA
To investigate whether the Tan1 protein catalyzed formation of ac4C in tRNA, a GST-tagged version of the protein was expressed and purified from E. coli (Fig. 4A ▶). Labeling experiments showed that both carbons of acetate were used to form the N4-acetyl group of ac4C, indicating that acetyl-coenzyme A (acetyl-CoA) is the donor in the modification reaction (Tumaitis and Lane 1970). The tagged protein was investigated for in vitro acetyltransferase activity by using T7 transcribed radiolabeled 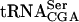 as substrate and acetyl-CoA as the donor. The samples were degraded to nucleotides and subjected to two-dimensional thin-layer chromatography. However, no formation of pac4C using the GST-Tan1 protein was detected (data not shown).
as substrate and acetyl-CoA as the donor. The samples were degraded to nucleotides and subjected to two-dimensional thin-layer chromatography. However, no formation of pac4C using the GST-Tan1 protein was detected (data not shown).
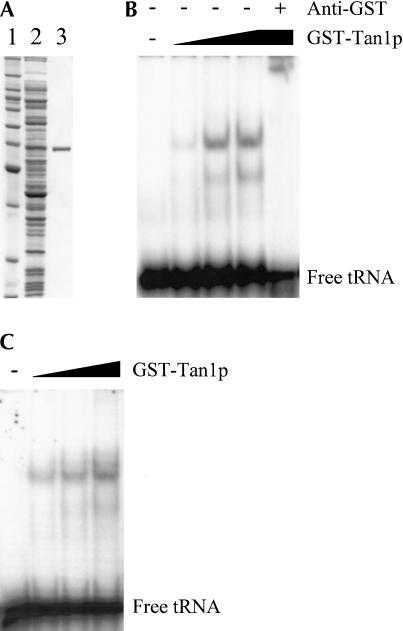
FIGURE 4.
The Tan1 protein interacts with tRNA. (A) SDS-PAGE analysis of GST-Tan1 protein purified from E. coli. The gel was stained with Coomassie brilliant Blue R-250. (Lane 1) Molecular weight standard (BenchMark protein ladder, Invitrogen). (Lane 2) E. coli crude extract with expressed GST-Tan1 protein. (Lane 3) Purified GST-Tan1 protein. (B) Gel mobility shift assay using 32P-labeled T7-transcribed 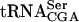 and increasing amounts (0.15, 0.3, and 0.6 μg) of GST-Tan1 protein. The supershift was induced by monoclonal anti-GST antibody. (C) Gel mobility shift assay using 32P-labeled T7-transcribed
and increasing amounts (0.15, 0.3, and 0.6 μg) of GST-Tan1 protein. The supershift was induced by monoclonal anti-GST antibody. (C) Gel mobility shift assay using 32P-labeled T7-transcribed 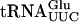 and increasing amounts (0.15, 0.3, and 0.6 μg) of GST-Tan1 protein.
and increasing amounts (0.15, 0.3, and 0.6 μg) of GST-Tan1 protein.
The Tan1 protein has been shown to contain a THUMP domain, a predicted RNA-binding motif shared by 4-thiouridine, pseudouridine synthases, and RNA methyltransferases (Aravind and Koonin 2001). To investigate whether Tan1p binds tRNA, a gel mobility shift assay was performed. Radiolabeled T7 transcribed 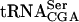 or
or 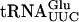 was mixed with increasing amounts of GST-Tan1 protein. The ac4C nucleoside is normally present in
was mixed with increasing amounts of GST-Tan1 protein. The ac4C nucleoside is normally present in 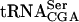 (Etcheverry et al. 1979), but not in
(Etcheverry et al. 1979), but not in 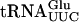 , which has a G at position 12 (Kobayashi et al. 1974). As a control, purified GST protein was mixed with radiolabeled tRNA. Analysis by native PAGE revealed an ability of GST-Tan1p, but not GST alone, to shift the mobility of
, which has a G at position 12 (Kobayashi et al. 1974). As a control, purified GST protein was mixed with radiolabeled tRNA. Analysis by native PAGE revealed an ability of GST-Tan1p, but not GST alone, to shift the mobility of 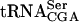 (Fig. 4B ▶; data not shown). Addition of an excess of total unlabeled tRNA prevented the mobility shift (data not shown). Although
(Fig. 4B ▶; data not shown). Addition of an excess of total unlabeled tRNA prevented the mobility shift (data not shown). Although 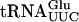 does not contain ac4C12, the presence of GST-Tan1p changed the mobility of this tRNA (Fig. 4C ▶). To verify that Tan1p and not a contaminant in the preparation was responsible for tRNA binding, a supershift experiment was performed in which radiolabeled
does not contain ac4C12, the presence of GST-Tan1p changed the mobility of this tRNA (Fig. 4C ▶). To verify that Tan1p and not a contaminant in the preparation was responsible for tRNA binding, a supershift experiment was performed in which radiolabeled 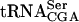 was incubated with GST-Tan1p in the presence of monoclonal anti-GST antibody. The anti-GST antibody caused a supershift of the
was incubated with GST-Tan1p in the presence of monoclonal anti-GST antibody. The anti-GST antibody caused a supershift of the 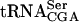 /protein complex (Fig. 4B ▶). These data show that Tan1p binds tRNA and indicate a direct role in synthesis of ac4C12.
/protein complex (Fig. 4B ▶). These data show that Tan1p binds tRNA and indicate a direct role in synthesis of ac4C12.
The TAN1 gene is required for stability of a mutant form of 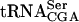
To investigate the molecular mechanism for the synthetic interaction of the tan1Δ and sup61-T47:2C alleles, we intended to determine the relative amount of 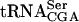 in the double mutant. Although the sup61-T47:2C tan1Δ strain was viable at 25°C, it was extremely slow growing and difficult to handle. To circumvent this problem, we constructed a sup61-T47:2C tan1Δ strain rescued by a plasmid carrying TAN1 and introduced a high copy plasmid carrying sup16+, encoding
in the double mutant. Although the sup61-T47:2C tan1Δ strain was viable at 25°C, it was extremely slow growing and difficult to handle. To circumvent this problem, we constructed a sup61-T47:2C tan1Δ strain rescued by a plasmid carrying TAN1 and introduced a high copy plasmid carrying sup16+, encoding 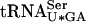 . This tRNA contains an uncharacterized modified uridine residue at position 34 that is likely to reduce wobble and thereby reading of UCG codons (Etcheverry et al. 1979, 1982). However, increased gene dosage of sup16+ suppresses the lethality of a sup61Δ mutant (M.J.O. Johansson and A.S. Byström, unpubl.), possibly by an ability of a pool of hypomodified
. This tRNA contains an uncharacterized modified uridine residue at position 34 that is likely to reduce wobble and thereby reading of UCG codons (Etcheverry et al. 1979, 1982). However, increased gene dosage of sup16+ suppresses the lethality of a sup61Δ mutant (M.J.O. Johansson and A.S. Byström, unpubl.), possibly by an ability of a pool of hypomodified 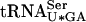 to decode UCG codons. Alternatively, an increased pool of fully modified
to decode UCG codons. Alternatively, an increased pool of fully modified 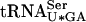 might be sufficient to suppress the need for
might be sufficient to suppress the need for 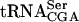 . Introduction of a high copy plasmid carrying sup16+ alleviated the requirement for the TAN1 plasmid in the sup61T47:2C tan1Δ strain at 30°C (Fig. 2B ▶).
. Introduction of a high copy plasmid carrying sup16+ alleviated the requirement for the TAN1 plasmid in the sup61T47:2C tan1Δ strain at 30°C (Fig. 2B ▶).
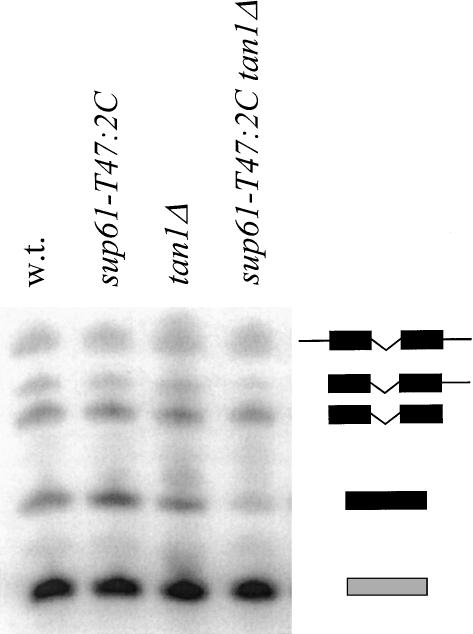
Total RNA was prepared from exponentially growing cultures, at 30°C, of wild-type, sup61-T47:2C, tan1Δ, and sup61-T47:2C tan1Δ strains carrying the high copy sup16+ plasmid. The relative amount of mature and precursor 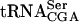 was determined by using Northern blot analysis. The sup61 gene contains an intron (Etcheverry et al. 1979) and two different 32P-labeled oligonucleotides were used to detect mature and pre-
was determined by using Northern blot analysis. The sup61 gene contains an intron (Etcheverry et al. 1979) and two different 32P-labeled oligonucleotides were used to detect mature and pre-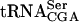 . Signals from the mature and the different pre-
. Signals from the mature and the different pre-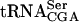 species (O’Connor and Peebles 1991; Yoo and Wolin 1997) were quantified and normalized to the initiator methionine tRNA (
species (O’Connor and Peebles 1991; Yoo and Wolin 1997) were quantified and normalized to the initiator methionine tRNA (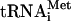 ) signal, which served as an internal control, as ac4C is not present in
) signal, which served as an internal control, as ac4C is not present in 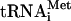 . The analysis revealed that the tan1Δ mutant showed essentially the same level of
. The analysis revealed that the tan1Δ mutant showed essentially the same level of 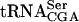 as the wild-type strain (Fig. 5 ▶; Table 1 ▶). However, the sup61-T47:2C tan1Δ mutant showed a decreased level of mature
as the wild-type strain (Fig. 5 ▶; Table 1 ▶). However, the sup61-T47:2C tan1Δ mutant showed a decreased level of mature 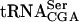 compared with the wild-type, sup61-T47:2C, and tan1Δ strains (Fig. 5 ▶; Table 1 ▶). The level of pre-
compared with the wild-type, sup61-T47:2C, and tan1Δ strains (Fig. 5 ▶; Table 1 ▶). The level of pre-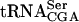 was similar in the wild-type, single mutant, and double mutant strains (Fig. 5 ▶; Table 1 ▶), indicating that ac4C12 and/or Tan1p is required for stability of mature
was similar in the wild-type, single mutant, and double mutant strains (Fig. 5 ▶; Table 1 ▶), indicating that ac4C12 and/or Tan1p is required for stability of mature 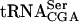 .
.
FIGURE 5.
The TAN1 gene product stabilizes the mutant form of 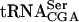 . Northern blot analysis of total RNA isolated from wild-type (UMY2220), sup61-T47:2C (UMY2256), tan1Δ (UMY2874), and sup61-T47:2C tan1Δ (derived from UMY2951) strains, carrying pRS425-sup16+, grown in SC-Leu medium at 30°C. The blot was probed simultaneously for pre-
. Northern blot analysis of total RNA isolated from wild-type (UMY2220), sup61-T47:2C (UMY2256), tan1Δ (UMY2874), and sup61-T47:2C tan1Δ (derived from UMY2951) strains, carrying pRS425-sup16+, grown in SC-Leu medium at 30°C. The blot was probed simultaneously for pre-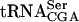 ,
, 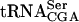 , and
, and 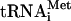 (see Materials and Methods). The position and identity of
(see Materials and Methods). The position and identity of 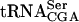 (black) and
(black) and 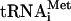 (gray) species are indicated on the right.
(gray) species are indicated on the right.
TABLE 1.
Effects of the tan1- null allele on the relative level of 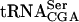
| tRNA species | Wild type | sup61-T47:2C | tan1Δ | sup61-T47:2C tan1Δ |
pre-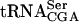
|
1 | 1.2 ± 0.1 | 1.2 ± 0.3 | 1.4 ± 0.5 |
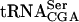 |
1 | 1.3 ± 0.4 | 0.8 ± 0.1 | 0.2 ± 0.1 |
Data from Fig. 5 ▶ and two additional independent experiments (data not shown) were averaged. The standard deviation is indicated. The level of pre-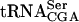 is the combined signal of the three precursor forms normalized to mature
is the combined signal of the three precursor forms normalized to mature 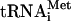 , relative to the corresponding value in the wild-type strain, which was set to 1. The normalized value for mature
, relative to the corresponding value in the wild-type strain, which was set to 1. The normalized value for mature 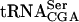 was expressed relative to the corresponding value in the wild-type stain, which was set to one.
was expressed relative to the corresponding value in the wild-type stain, which was set to one.
DISCUSSION
The modified nucleoside ac4C is present at position 12 in a subset of eukaryotic tRNAs. In this study, we describe the identification and characterization of the S. cerevisiae TAN1 gene encoding a protein required for formation of ac4C12. The gene was identified in a screen for mutations lethal in combination with a sup61-T47:2C allele, coding for a mutant form of 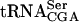 . Analysis of RNA from a tan1-null strain revealed that the modified nucleoside ac4C was absent in tRNA but not in rRNA (Fig. 3 ▶, data not shown). The absence of Tan1p in the sup61-T47:2C strain caused about a sixfold reduction in the level of mature
. Analysis of RNA from a tan1-null strain revealed that the modified nucleoside ac4C was absent in tRNA but not in rRNA (Fig. 3 ▶, data not shown). The absence of Tan1p in the sup61-T47:2C strain caused about a sixfold reduction in the level of mature 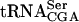 , whereas the level of pre-
, whereas the level of pre-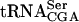 was relatively unaffected (Fig. 5 ▶, Table 1 ▶). This suggested that the acetylation of C12 and/or the Tan1p stabilized the mutant tRNA following removal of the 5′ leader, 3′ trailer, and intervening sequences in pre-
was relatively unaffected (Fig. 5 ▶, Table 1 ▶). This suggested that the acetylation of C12 and/or the Tan1p stabilized the mutant tRNA following removal of the 5′ leader, 3′ trailer, and intervening sequences in pre-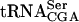 .
.
We were unable to detect any in vitro tRNA acetyltransferase activity by a GST-Tan1 fusion protein. It was unlikely that the GST moiety influenced the catalytic activity, as a GST-Tan1 protein expressed in yeast complemented the absence of ac4C in the null mutant (data not shown). The Tan1 protein contains a THUMP domain proposed to be an RNA-binding motif shared by a number of modification enzymes (Aravind and Koonin 2001). By using a gel-shift assay, GST-Tan1p was found to interact with 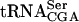 , suggesting that Tan1p is directly involved in synthesis of ac4C12 (Fig. 4B ▶). It is possible that Tan1p functions as the tRNA binding component of a modification enzyme consisting of more than one subunit. This would explain the absence of acetyltransferase activity by the purified GST-Tan1 protein. Consistent with this hypothesis, Tan1p does not show any apparent homology with known acetyltransferases. There are three examples in S. cerevisiae of tRNA modification enzymes consisting of two different subunits, Gcd10/Gcd14 (Anderson et al. 2000), Tad2/Tad3 (Gerber and Keller 1999), and Trm8/Trm82 (Alexandrov et al. 2002). An additional subunit(s) could be identified in the synthetic lethal screen, assuming that the mutant form of
, suggesting that Tan1p is directly involved in synthesis of ac4C12 (Fig. 4B ▶). It is possible that Tan1p functions as the tRNA binding component of a modification enzyme consisting of more than one subunit. This would explain the absence of acetyltransferase activity by the purified GST-Tan1 protein. Consistent with this hypothesis, Tan1p does not show any apparent homology with known acetyltransferases. There are three examples in S. cerevisiae of tRNA modification enzymes consisting of two different subunits, Gcd10/Gcd14 (Anderson et al. 2000), Tad2/Tad3 (Gerber and Keller 1999), and Trm8/Trm82 (Alexandrov et al. 2002). An additional subunit(s) could be identified in the synthetic lethal screen, assuming that the mutant form of 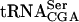 requires presence of ac4C12 and not the Tan1 protein per se. However, it cannot be excluded that the lack of in vitro acetyltransferase activity by GST-Tan1p was caused by unfavorable assay conditions. In addition to the
requires presence of ac4C12 and not the Tan1 protein per se. However, it cannot be excluded that the lack of in vitro acetyltransferase activity by GST-Tan1p was caused by unfavorable assay conditions. In addition to the 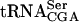 interaction, Gst-Tan1p was found to bind
interaction, Gst-Tan1p was found to bind 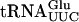 (Fig. 4C ▶), which has a G at position 12. It is not clear whether the interaction with
(Fig. 4C ▶), which has a G at position 12. It is not clear whether the interaction with 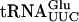 is relevant in vivo, that is, whether Tan1p is involved in maturation of tRNAs not containing ac4C12. Although only tRNAs specific for serine and leucine contain ac4C12, the Tan1 protein might recognize general tRNA structure. It has previously been shown that the E. coli tRNA(m1G37) methyltransferase in vitro binds tRNA species that are not methylated by the enzyme (Redlak et al. 1997). Alternatively, specificity in RNA binding by Tan1p might be provided by an additional subunit(s).
is relevant in vivo, that is, whether Tan1p is involved in maturation of tRNAs not containing ac4C12. Although only tRNAs specific for serine and leucine contain ac4C12, the Tan1 protein might recognize general tRNA structure. It has previously been shown that the E. coli tRNA(m1G37) methyltransferase in vitro binds tRNA species that are not methylated by the enzyme (Redlak et al. 1997). Alternatively, specificity in RNA binding by Tan1p might be provided by an additional subunit(s).
To date, six different gene products have been identified to be important for growth of strains with a mutant form of 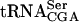 (this study, Yoo and Wolin 1997; Johansson and Byström 2002). All of these gene products interact with and influence stability of mutant forms of
(this study, Yoo and Wolin 1997; Johansson and Byström 2002). All of these gene products interact with and influence stability of mutant forms of 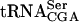 . Thus, the strains in remaining 10 complementation groups identified in the screen are likely to carry mutations in genes coding for products that directly or indirectly influence
. Thus, the strains in remaining 10 complementation groups identified in the screen are likely to carry mutations in genes coding for products that directly or indirectly influence 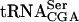 stability. In conclusion, the screen for mutations lethal in combination with a sup61 allele is an excellent genetic method to identify gene products that participate in
stability. In conclusion, the screen for mutations lethal in combination with a sup61 allele is an excellent genetic method to identify gene products that participate in 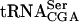 maturation. It should be possible to identify gene products specific for other tRNA species using an analogous approach.
maturation. It should be possible to identify gene products specific for other tRNA species using an analogous approach.
MATERIALS AND METHODS
Strains, media, and genetic procedures
The source and genotypes of yeast strains used in this study are listed in Table 2 ▶. E. coli strains used were DH5α (Bethesda Research Laboratories) and BL21 (Studier and Moffatt 1986). Yeast transformation (Gietz et al. 1992), media, and genetic procedures have been described (Burke et al. 2000). Strain UMY2704 was obtained from a tetrad in a cross between UMY2256, carrying pMJ1421, and UMY2220. Plasmid pRS304-TAN1 was linearized with BglII and targeted (Orr-Weaver et al. 1981) to the TAN1 locus in strain UMY2256 generating UMY2871. A tan1 : : KanMX4 construct was PCR amplified from a homozygous tan1 : : KanMX4 (ygl232w : : KanMX4) diploid strain (34599, Research Genetics) and transformed into UMY2366, which is a diploid strain formed between UMY2219 and UMY2220. The generated heterozygous diploid was allowed to sporulate and the tan1-null mutant (UMY2874) obtained from a tetrad. Strain UMY2951 was obtained from a tetrad in a cross between UMY2256, carrying pMJ1422, and UMY2874. The pMJ1422 plasmid in UMY2951 was replaced with pRS316-TAN1 generating the strain used in Figure 2 ▶.
TABLE 2.
S. cerevisiae strains used in this study
| Yeast strain | Genotype | Source or reference |
| UMY2219 | MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG | this laboratory |
| UMY2220 | MATα ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG | this laboratory |
| UMY2366 | MATa/MATα ura3-1/ura3-1 leu2-3,112/leu2-3,112 trp1-1/trp1-1 his3-11,15/his3-11,15 can1-100/can1-100 ade2-1/ade2-1 ade3 : : hisG/ade3 : : hisG | this study |
| UMY2256 | MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG sup61-T47:2C | (Johansson and Byström 2002) |
| UMY2704 | MATα ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG sup61-T47:2C pMJ1421 | this study |
| UMY2871 | MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG sup61-T47:2C TAN1 : : pRS304-TAN1 | this study |
| UMY2874 | MATα ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG tan1 : : KanMX4 | this study |
| UMY2951 | MATα ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1 ade3 : : hisG sup61-T47:2C tan1 : : KanMX4 pMJ1422 | this study |
| 34599 | MATa/MATα ura3 Δ0/ura3Δ0 leu2 Δ0/leu2Δ0 his3Δ1/his3Δ1 met15Δ0/MET15 lys2Δ0/LYS2 ygl232w : : KanMX4/ygl232w : : KanMX4 | Research Genetics |
Synthetic lethal screen and identification of the TAN1 gene
The screen used to isolate mutants was based on colony sectoring as described previously (Kranz and Holm 1990; Bender and Pringle 1991). Strain UMY2704, carrying plasmid pMJ1421 bearing the sup61+, ADE3, and URA3 genes, was grown in selective medium to ~2 × 107 cells/mL. The cells were diluted with water, plated on YEPD plates, mutagenized with UV irradiation to ~20% survival, and incubated at 30°C. Uniformly red colonies were restreaked at least twice, and only those strains that continued to give nonsectored colonies were studied further. The candidate strains were transformed with pRS414-sup61+ (p1229) and strains that regained the ability to sector were crossed to UMY2256 to test for dominance/recessiveness and for 2:2 segregation of the nonsectoring phenotype.
A low copy genomic library in YCp50 (Rose et al. 1987) was transformed into one of the mutants, carrying pMJ1422, and transformants selected on medium lacking uracil. Plasmids from transformants that regained the ability lose the sup61+ vector were isolated and retransformed, and those that stayed positive were partially sequenced and subjected to homology searches against the S. cerevisiae genome. To investigate linkage between the TAN1 gene and the mutation, UMY2871 was crossed to each mutant, and tetrad analysis revealed that the sectoring phenotype cosegregated with the integrated plasmid marker (TRP1).
Plasmid constructions
DNA manipulations, plasmid preparations, and bacterial transformations were performed according to standard protocols. Plasmid pMJ1421 was constructed by cloning a SalI/SmaI sup61+ fragment from pRS316-sup61+ (Johansson and Byström 2002) into the SalI/NruI sites of plasmid c2013 (Cvrckova and Nasmyth 1993), removing the spADH : : CLN2 construct. The TRP1 variant of the ADE3 sup61+ plasmid (pMJ1422) was created by transforming a yeast strain carrying pMJ1421 with a ura3 : : TRP1 construct. The plasmid was isolated from a Trp+ Ura− strain and confirmed by restriction analysis. A low copy TRP1 vector carrying the sup61+ gene (p1229) was constructed by cloning a BglII/EcoRI sup61+ fragment from pRS316-sup61+ into the BamHI/EcoRI sites of pRS414 (Sikorski and Hieter 1989). Plasmids pRS304-TAN1 (p1482) and pRS316-TAN1 (p1483) were constructed by cloning an EcoRI/XhoI fragment, PCR amplified from the complementing library plasmid, into the corresponding sites of pRS304 and pRS316 (Sikorski and Hieter 1989). The oligonucleotides used were 5′-TTGAATTCTGTAGAAGGAGCTAACGGCGA-3′ and 5′TTCTCGAGCAATGGGTCTCATTCCTAACG-3′. The TAN1 gene contains an intron (Davis et al. 2000) and to express and purify the protein, the intron in plasmid pRS304-TAN1 was removed by PCR mutagenesis, generating pRS304-TAN1Δintron (p1547). The intronless gene was amplified and cloned as an EcoRI/XhoI fragment into pGEX-4T-2 (Amersham Biosciences) generating pGEX-4T-2-TAN1Δintron (p1612). In this plasmid, transcription is under the tac promoter and the product is a GST-Tan1 fusion protein. The oligonucleotides used to amplify the intronless gene were 5′-AAGAATTCCCATGGGTGAAAAACGTAAC-3′ and 5′-TTCTC GAGCAATGGGTCTCATTCCTAACG-3′. An intronless sup61+ gene under the T7 promoter was constructed by ligating three pairs of oligonucleotides into the EcoRI/BamHI sites of pUC18 (Roche Applied Science) generating pUC18-T7sup61+ΔIVS (p1611). The oligonucleotides used were 5′-AATTGCTGCAGT AATACGAC-3′, 5′-TATAGTGAGTCGTATTACTGCAGC-3′, 5′-TCACTATAGGCACTATGGCCGAGTGGTTAAGGCGAGAGAC TCGAAATC-3′, 5′-GCCCAAGAGATTTCGAGTCTCTCGCCTT AACCACTCGGCCATAGTGCC-3′, 5′-TCTTGGGCTCTGCCCG CGCTGGTTCAAATCCTGCTGGTGTCGCCAG-3′, and 5′-GAT CCTGGCGACACCAGCAGGATTTGAACCAGCGCGGGCAGA3′. Plasmids p1477, a pRS425 vector (Christianson et al. 1992) carrying the wild-type sup16+ gene, and p1536, a pUC18 plasmid carrying a 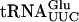 gene under the T7 promoter, were obtained from Bo Huang (this laboratory). The
gene under the T7 promoter, were obtained from Bo Huang (this laboratory). The 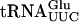 gene carried two mutations (G1-C72), to improve the efficiency of T7 transcription.
gene carried two mutations (G1-C72), to improve the efficiency of T7 transcription.
Purification of the GST-Tan1 protein
To purify the Tan1 protein, the bacterial strain BL21 (Studier and Moffatt 1986) was transformed with pGEX-4T-2-TAN1Δintron. A total volume of 25 mL LB medium containing 50 μg/mL carbenicillin was inoculated and grown at 25°C to OD600 = 0.5 before IPTG was added to a final concentration of 0.1 mM. The cells were harvested after 2 h at 25°C, dissolved in PBS, and disrupted by sonication. The GST-Tan1 protein was batch-purified using glutathione Sepharose 4B according to manufacturer’s instructions (Amersham Biosciences). Purification of GST protein was performed in an identical manner by using BL21 transformed with the pGEX-4T-2 vector.
RNA methods
Total RNA for Northern blots was prepared by using glass beads essentially as described (Ausubel et al. 2001). For Northern blot analysis ~10μg total RNA was separated on 8% polyacrylamide, 8 M urea gels and transferred to Zeta-Probe membranes (Bio-Rad). Oligonucleotides used to detect precursor and mature 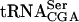 were 5′- AGCCGAACTTTTTATTCCATTCG-3′ and 5′-GCCCA AGAGATTTCGAGTCTCT-3′, respectively. To detect
were 5′- AGCCGAACTTTTTATTCCATTCG-3′ and 5′-GCCCA AGAGATTTCGAGTCTCT-3′, respectively. To detect 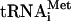 , the oligonucleotide 5′-GGACATCAGGGTTATGAGCC-3′ was used. Oligonucleotides were labeled by using 5′[γ32P]ATP (5000 Ci/mmole, Amersham Biosciences) and polynucleotide kinase (Roche Applied Science). Northern blots were visualized and quantified by PhosphorImager analysis.
, the oligonucleotide 5′-GGACATCAGGGTTATGAGCC-3′ was used. Oligonucleotides were labeled by using 5′[γ32P]ATP (5000 Ci/mmole, Amersham Biosciences) and polynucleotide kinase (Roche Applied Science). Northern blots were visualized and quantified by PhosphorImager analysis.
The T7-transcribed radiolabeled 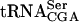 and
and 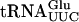 was prepared by using MvaI linearized pUC18-T7sup61+ΔIVS or p1536, 5′[α32P]CTP or 5′[α32P]UTP (400 Ci/mmole, Amersham Biosciences) and the Riboprobe in vitro transcription system (Promega). The radiolabeled transcripts were purified on an 8% polyacrylamide and 8 M urea gel. Transfer RNA was eluted from gel slices in 2 M ammonium acetate, 1% SDS followed by precipitation. The tRNA pellet was dissolved in water. Gel mobility shift assays was performed essentially as described previously (Kambampati and Lauhon 2000) by incubating 32P-labeled
was prepared by using MvaI linearized pUC18-T7sup61+ΔIVS or p1536, 5′[α32P]CTP or 5′[α32P]UTP (400 Ci/mmole, Amersham Biosciences) and the Riboprobe in vitro transcription system (Promega). The radiolabeled transcripts were purified on an 8% polyacrylamide and 8 M urea gel. Transfer RNA was eluted from gel slices in 2 M ammonium acetate, 1% SDS followed by precipitation. The tRNA pellet was dissolved in water. Gel mobility shift assays was performed essentially as described previously (Kambampati and Lauhon 2000) by incubating 32P-labeled 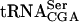 or
or 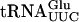 (1 to 2 ng) with varying amounts of GST or GST-Tan1 proteins for 10 min at 30°C before applying the samples to a native 8% polyacrylamide gel. The monoclonal anti-glutathione-s-transferase (GST) antibody used for the supershift experiment was purchased from Sigma.
(1 to 2 ng) with varying amounts of GST or GST-Tan1 proteins for 10 min at 30°C before applying the samples to a native 8% polyacrylamide gel. The monoclonal anti-glutathione-s-transferase (GST) antibody used for the supershift experiment was purchased from Sigma.
Total tRNA for HPLC analysis was prepared essentially as described (Björk et al. 2001) followed by LiCl fractionation (Avital and Elson 1969). For HPLC analysis 50 μg tRNA was digested to nucleosides by using nuclease P1 and bacterial alkaline phosphatase (Gehrke et al. 1982), and the hydrolysate was analyzed by HPLC (Gehrke and Kuo 1990). N4-acetylcytidine was purchased from Sigma.
Acknowledgments
Dr. G.R. Björk, Dr. T.G. Hagervall, and A. Esberg are acknowledged for comments on the manuscript. Kerstin Jacobsson is acknowledged for technical assistance. This work was financially supported by the Swedish Research Council (621-2001-1890) and the Swedish Cancer Society (3516-B01-08XAB).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5198204.
REFERENCES
- Alexandrov, A., Martzen, M.R., and Phizicky, E.M. 2002. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA 8: 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J., Phan, L., and Hinnebusch, A.G. 2000. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. 97: 5173–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind, L. and Koonin, E.V. 2001. THUMP: A predicted RNA-binding domain shared by 4-thiouridine, pseudouridine synthases and RNA methylases. Trends Biochem. Sci. 26: 215–217. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K., eds. 2001. Current protocols in molecular biology. John Wiley and Sons, New York.
- Avital, S. and Elson, D. 1969. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim. Biophys. Acta 179: 297–307. [DOI] [PubMed] [Google Scholar]
- Becker, H.F., Motorin, Y., Planta, R.J., and Grosjean, H. 1997. The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalyzing the formation of psi55 in both mitochondrial and cytoplasmic tRNAs. Nucleic Acids Res. 25: 4493–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A. and Pringle, J.R. 1991. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, G.R. 1986. Transfer RNA modifications in different organism. Chemica Scripta 26B: 91–95. [Google Scholar]
- Björk, G.R., Jacobsson, K., Nilsson, K., Johansson, M.J.O., Byström, A.S., and Persson, O.P. 2001. A primordial tRNA modification required for the evolution of life? EMBO J. 20: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke, J.D., LaCroute, F., and Fink, G.R. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197: 345–346. [DOI] [PubMed] [Google Scholar]
- Burke, D., Dawon, D., and Stearns, T. 2000. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Cavaille, J., Chetouani, F., and Bachellerie, J.P. 1999. The yeast Saccharomyces cerevisiae YDL112w ORF encodes the putative 2′-o-ribose methyltransferase catalyzing the formation of Gm18 in tRNAs. RNA 5: 66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian, N. and Cedergren, R. 1998. Modification nucleotides always were: An evolutionary model. In Modification and editing of RNA (eds. H. Grosjean and R. Benne), pp. 535–541. ASM Press, Washington, DC.
- Christianson, T.W., Sikorski, R.S., Dante, M., Shero, J.H., and Hieter, P. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. [DOI] [PubMed] [Google Scholar]
- Cvrckova, F. and Nasmyth, K. 1993. Yeast G1 cyclins CLN1 and CLN2 and a GAP-like protein have a role in bud formation. EMBO J. 12: 5277–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C.A., Grate, L., Spingola, M., and Ares Jr., M. 2000. Test of intron predictions reveals novel splice sites, alternatively spliced mRNAs and new introns in meiotically regulated genes of yeast. Nucleic Acids Res. 28: 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, S.R., Morales, M.J., Li, J.M., Hopper, A.K., and Martin, N.C. 1986. Isolation and characterization of the TRM1 locus, a gene essential for the N2,N2-dimethylguanosine modification of both mitochondrial and cytoplasmic tRNA in Saccharomyces cerevisiae. J. Biol. Chem. 261: 9703–9709. [PubMed] [Google Scholar]
- Etcheverry, T., Colby, D., and Guthrie, C. 1979. A precursor to a minor species of yeast tRNASer contains an intervening sequence. Cell 18: 11–26. [DOI] [PubMed] [Google Scholar]
- Etcheverry, T., Salvato, M., and Guthrie, C. 1982. Recessive lethality of yeast strains carrying the SUP61 suppressor results from loss of a transfer RNA with a unique decoding function. J. Mol. Biol. 158: 599–618. [DOI] [PubMed] [Google Scholar]
- Gehrke, C.W. and Kuo, K.C. 1990. Chromatography and modification of nucleosides. Elsevier, Amsterdam.
- Gehrke, C.W., Kuo, K.C., McCune, R.A., Gerhardt, K.O., and Agris, P.F. 1982. Quantitative enzymatic hydrolysis of tRNAs: Reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 230: 297–308. [PubMed] [Google Scholar]
- Gerber, A.P. and Keller, W. 1999. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 286: 1146–1149. [DOI] [PubMed] [Google Scholar]
- Giaever, G., Chu, A.M., Ni, L., Connelly, C., Riles, L., Veronneau, S., Dow, S., Lucau-Danila, A., Anderson, K., Andre, B., et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. [DOI] [PubMed] [Google Scholar]
- Gietz, D., St. Jean, A., Woods, R.A., and Schiestl, R.H. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper, A.K. and Phizicky, E.M. 2003. tRNA transfers to the limelight. Genes & Dev. 17: 162–180. [DOI] [PubMed] [Google Scholar]
- Johansson, M.J.O. and Byström, A.S. 2002. Dual function of the tRNA(m5U54)methyltransferase in tRNA maturation. RNA 8: 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambampati, R. and Lauhon, C.T. 2000. Evidence for the transfer of sulfane sulfur from IscS to ThiI during the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. J. Biol. Chem. 275: 10727–10730. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Irie, T., Yoshida, M., Takeishi, K., and Ukita, T. 1974. The primary structure of yeast glutamic acid tRNA specific to the GAA codon. Biochim. Biophys. Acta 366: 168–181. [DOI] [PubMed] [Google Scholar]
- Kranz, J.E. and Holm, C. 1990. Cloning by function: an alternative approach for identifying yeast homologs of genes from other organisms. Proc. Natl. Acad. Sci. 87: 6629–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund, M.E., Johansson, J.O.M., von Pawel-Rammingen, U., and Byström, A.S. 2000. Identification of the TRM2 gene encoding the tRNA(m5U54)methyltransferase of Saccharomyces cerevisiae. RNA 6: 844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor, J.P. and Peebles, C.L. 1991. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver, T.L., Szostak, J.W., and Rothstein, R.J. 1981. Yeast transformation: A model system for the study of recombination. Proc. Natl. Acad. Sci. 78: 6354–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlak, M., Andraos-Selim, C., Giege, R., Florentz, C., and Holmes, W.M. 1997. Interaction of tRNA with tRNA (guanosine-1)methyltransferase: Binding specificity determinants involve the dinucleotide G36pG37 and tertiary structure. Biochemistry 36: 8699–8709. [DOI] [PubMed] [Google Scholar]
- Rose, M.D., Novick, P., Thomas, J.H., Botstein, D., and Fink, G.R. 1987. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60: 237–243. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S. and Hieter, P. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl, M., Horn, C., Brown, M., Ioudovitch, A., and Steinberg, S. 1998. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 26: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, L. and Schulman, L.H. 1978. The role of the minor base N4-acetylcytidine in the function of the Escherichia coli noninitiator methionine transfer RNA. J. Biol. Chem. 253: 6132–6139. [PubMed] [Google Scholar]
- Studier, F.W. and Moffatt, B.A. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189: 113–130. [DOI] [PubMed] [Google Scholar]
- Thomas, G., Gordon, J., and Rogg, H. 1978. N4-Acetylcytidine: A previously unidentified labile component of the small subunit of eukaryotic ribosomes. J. Biol. Chem. 253: 1101–1105. [PubMed] [Google Scholar]
- Tumaitis, T.D. and Lane, B.G. 1970. Differential labelling of the carboxymethyl and methyl substituents of 5-carboxymethyluridine methyl ester, a trace nucleoside constituent of yeast transfer RNA. Biochim. Biophys. Acta 224: 391–403. [DOI] [PubMed] [Google Scholar]
- Yoo, C.J. and Wolin, S.L. 1997. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell 89: 393–402. [DOI] [PubMed] [Google Scholar]