Abstract
Argonaute (Ago) proteins are the effector proteins of RNA interference (RNAi) and related silencing mechanisms that are mediated by small RNAs. Ago proteins bind directly to microRNAs (miRNAs) and to short interfering RNAs and are the core protein components of RNA induced silencing complexes (RISCs) and microRNPs (miRNPs). Here we report that an ~70-nt RNA associates specifically with immunopurified Ago2 expressed in human 293 cells. By directional cloning we identified this RNA as the mitochondrial tRNAMet (mt tRNAMet). Various exported (mt) tRNAs were detected in the cytosol of 293 cells, but Ago2 was found selectively bound to (mt) tRNAMet. The association in the cytosol of exported (mt) tRNAMet with Ago2 complements genetic and microscopic data that link mitochondria with RNAi-related components and events.
Keywords: Argonaute, mitochondria, tRNA, microRNA, miRNP, RISC
Argonaute (Ago) proteins are found in most organisms from fission yeast to humans (Carmell et al. 2002). Ago proteins play key roles in all RNA interference (RNAi)- related phenomena, including microRNA (miRNA) or short interfering RNA (siRNA)-directed target RNA cleavage, translational repression, or chromatin silencing (Carmell et al. 2002; Lippman and Martienssen 2004; Meister and Tuschl 2004; Murchison and Hannon 2004). Mammals have four canonical Ago proteins (Ago1–4), all of which associate with miRNAs and siRNAs (Liu et al. 2004; Meister et al. 2004). Mammalian Ago2 is the endonuclease that cleaves RNA targets that bear extensive complementarity to miRNAs or to siRNAs (Liu et al. 2004; Meister et al. 2004; Song et al. 2004). miRNAs are the major RNA species associated with human Ago2 when immunoprecipitated with an anti-Ago2 monoclonal antibody (Mourelatos et al. 2002). Mammalian Ago2 is localized predominantly in the cytoplasm and in association with the endoplasmic reticulum and Golgi apparatus in rat kidney cells (Cikaluk et al. 1999).
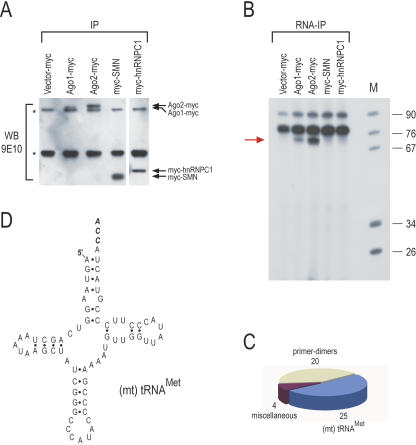
To study further Ago-associated RNAs, we transfected human embryonic kidney 293T cells with mouse Ago2 and Ago1 that carried a carboxy-terminal, myc epitope tag (Doi et al. 2003) and performed immunoprecipitations with the anti-myc antibody 9E10. Immunoprecipitations from 293T cells, transfected with vector only or with two other myc-tagged, RNA binding proteins, SMN and hnRNPC1, served as negative controls. We first analyzed the immunoprecipitates by Western blots with 9E10. As shown in Figure 1A, all transfected proteins were expressed and immunoprecipitated, although the expression of Ago1-myc was lower than that of Ago2-myc. Next, RNA was isolated from the immunoprecipitates, 3′-end labeled with [5′-32P]-pCp, and resolved on 10% UREA-PAGE. As shown in Figure 1B, an abundant ~70-nt RNA co-immunoprecipitated specifically with Ago2-myc. The ~70-nt RNA was also detected in Ago1-myc immunoprecipitates (Fig. 1B) but was less abundant, likely because the amount of immunoprecipitated Ago1-myc protein was less than the amount of Ago2-myc protein (Fig. 1A). Interestingly, ~22-nt RNAs, corresponding to miRNAs, were mostly absent from Ago2-myc or Ago1-myc immunoprecipitates (Fig. 1B), although we did detect low amounts of miRNAs in some of these experiments. However, the ~70-nt band was consistently present in all experiments and was therefore gel excised and directionally cloned. The statistics of the cloning are presented in Figure 1C. Forty-nine clones were sequenced, and of those, 25 clones contained inserts corresponding to the human mitochondrial (mt) tRNAMet (Fig. 1D). Many of these clones contained the full-length sequence of (mt) tRNAMet, including the CCA triplet at the 3′ end, which is added post-transcriptionally. All human (mt) tRNAs, including (mt) tRNAMet, are transcribed from mitochondrial DNA (Wallace 1999). There are four pseudogenes of (mt) tRNAMet in the human genome, but they all contain mutations that distinguish them from the authentic (mt) tRNAMet. Because all the clones that we sequenced from the Ago2 immunoprecipitates corresponded to the authentic (mt) tRNAMet sequence, the (mt) tRNAMet that associates with Ago2 is derived from mitochondria.
FIGURE 1.
Cloning of the (mt) tRNAMet from immunoprecipitates of myc-tagged Ago2. (A) Immunoprecipitations (IP) with 9E10 (anti-myc) antibody were performed from human 293T cells that had been transfected with constructs expressing the indicated proteins. Immunoprecipitates were analyzed by Western blot with 9E10. myc-Tagged proteins are indicated with arrows. Asterisks indicate antibody chains. (B) RNA was isolated from the indicated immunoprecipitates, 3′-end labeled withT4 RNA ligase, and [5′-32P]-pCp and resolved on 10%UREA-PAGE. The ~70-nt RNA that associated specifically with Ago2-myc (red arrow) was directionally cloned. (C) Results of cloning. (D) Secondary structure of the human (mt) tRNAMet. The post-transcriptionally added CCA triplet is shown in italics.
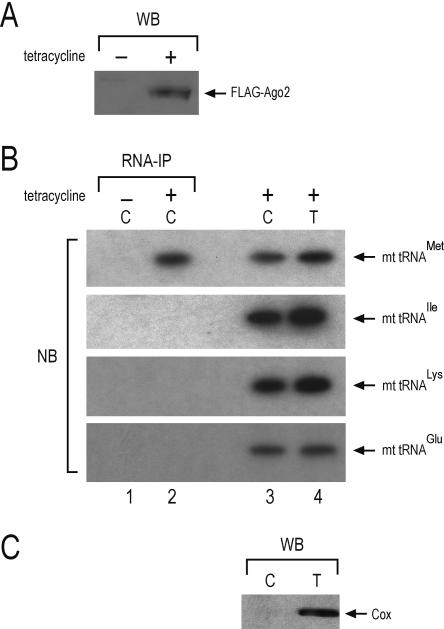
We considered the possibility that the association of (mt) tRNAMet with Ago2might represent an overexpression artifact or that it might have occurred as a result of contamination of the lysate with mitochondrial components during cell lysis. We also considered the possibility that placement of the myc tag at the carboxy terminus of Ago2might have altered the properties of the protein. To eliminate these possibilities we performed immunoprecipitations from cytosolic lysates, devoid of mitochondria, from a 293 cell line that expressed stablyFLAG-Ago2. First, we cloned Ago2 with a FLAG tag at the amino terminus and we used the Flp-In system (Invitrogen) to create a stable 293 cell line in which the FLAG-Ago2 transgene was integrated in a defined genomic locus as a single copy and under the control of a tetracycline inducible promoter. We then lysed the cells using conditions that preserved the integrity of the mitochondria and prepared cytosolic lysates devoid of mitochondria using the ApoAlert Cell Fractionation Kit (BD-Biosciences/Clontech) (Dounce et al. 1955). We analyzed the lysates prepared from induced and uninduced 293 cells on a Western blot using an anti-FLAG antibody. As shown in Figure 2A, FLAG-Ago2 was present only in the lysate from the tetracycline induced 293 cells. We then performed immunoprecipitations with anti-FLAG antibody from cytosolic lysates (devoid of mitochondria) derived from the tetracycline induced and from the uninduced 293 cells. We isolated RNA from the immunoprecipitates and analyzed it by Northern blotting using radiolabeled probes specific for four (mt) tRNAs: tRNAMet, tRNAIle, tRNALys, and tRNAGlu. We also analyzed the cytosolic lysate (devoid of mitochondria) and the total lysate (that contained mitochondria) from the tetracycline induced 293 cells. As shown in Figure 2B, only the (mt) tRNAMet associated with FLAG-Ago2, although all tested (mt) tRNA were present in the cytosol of 293 cells. Further more we analyzed the cytosolic lysates of the tetracycline induced 293 cells by Western blot using an antibody against the mitochondrial protein COX4. As shown in Figure 2C, COX4 was not detected in the cytosolic lysates, confirming that these lysates were devoid of mitochondria.
FIGURE 2.
Specific, cytoplasmic association of FLAG-Ago2 with exported (mt) tRNAMet. (A) A 293 cell line was prepared that expresses FLAG-Ago2 after tetracycline induction. Lysates from non-induced (−) and from tetracycline induced (+) 293 cells were probed on a Western blot with anti-FLAG antibody. (B) Cytosolic lysates were prepared from uninduced and from tetracycline-induced cells. The cells were lysed under conditions that preserve the mitochondrial integrity and the lysates were spun to pellet the mitochondria. The post-mitochondrial supernatants (cytosolic lysates) were subjected to immunoprecipitation (IP) with anti-FLAG antibodies. RNA was isolated, resolved on a 10% UREA-PAGE, blotted on a membrane, and probed with radiolabled oligos against the indicated (mt) tRNAs. (Lane 1) Cytosolic IP from uninduced 293 cells; (lane 2) cytosolic IP from tetracycline-induced 293 cells. The blot also contains total RNA isolated from cytosolic lysates (C, lane 3) and from total lysates (T, lane 4) of tetracycline-induced cells. (C) The cytosolic lysate (C) and the total lysate (T), from tetracycline-induced cells, were analyzed by Western blot with an antibody against the mitochondrial COX4 protein. COX4 is not detected in the cytosolic lysate, confirming the absence of mitochondrial contamination.
The association of (mt) tRNAMet with Ago2 is intriguing. It is possible that (mt) tRNAMet plays a role, perhaps regulatory, in the assembly or function of RNA induced silencing complexes or microRNPs. Alternatively, Ago2 may have a mitochondrial function. Experiments to address the functional significance of the association between Ago2 and (mt) tRNAMet are in progress. Interestingly, a number of reports suggest a possible link between mitochondrial components and RNAi-related silencing mechanisms. Polar granules in Drosophila melanogaster apparently contain exported, mitochondrial small and large ribosomal subunits (Kobayashi et al. 1993) as well as the Argonaute protein Aubergine (Harris and Macdonald 2001). aubergine is required for pole cell formation (Harris and Macdonald 2001) and likely for silencing of transposable elements and other heterochromatic repeats (Aravin et al. 2001, 2004; Reiss et al. 2004). It is also notable that 10 out of 27 genes that were identified in a genetic screen in Caenorhabditis elegans as required for transposon silencing are genes with known mitochondrial functions (Vastenhouw et al. 2003). It is tempting to speculate that there might be cross talk between mitochondria and RNAi and that (mt) tRNAMet may provide a physical link that would allow such communication.
MATERIALS AND METHODS
Generation of a stable 293 cell line expressing FLAG-Ago2
The Ago2 insert was excised from the Ago-myc vector by digestion with BamHI and NotI and was ligated at the same restriction sites into a modified pcDNA3 vector, in frame with an amino-terminal FLAG epitope tag. The FLAG-Ago2-pcDNA3 plasmid was then digested with NotI, treated with mung bean nuclease to create a blunt end, and digested again with HindIII. The FLAG-Ago2 insert was gel purified and was ligated into a pcDNA5/FRT/TO vector (Invitrogen), which had been previously digested with NotI, followed by treatment with mung bean nuclease and a second digestion with HindIII. The resultant plasmid was transfected in Flp-In, 293 T-REx cells (Invitrogen) and a stable cell line was generated, according to the manufacturer’s instruction. The resultant stable 293 cell line contains a FLAG-Ago2 transgene that, through the Flp recombinase, has been integrated as a single copy in a defined genomic locus. Furthermore, the expression of FLAG-Ago2 is inducible by tetracycline.
Cell culture, immunoprecipitations, Western blots, RNA isolation and cloning, and Northern blots
These were performed as previously described (Mourelatos et al. 2002; Nelson et al. 2004). The sequences of the antisense oligo-nucleotide probes used on Northern blots to detect (mt) tRNAs were 5′-gtataaccaacattttcggggtatggg (tRNAMet); 5′-gggtttaagctcc tattatttactctatcaaag (tRNAIle); 5′-ggtgttggttctcttaatctttaacttaaaagg (tRNALys); and 5′-cta caaccacgaccaatgatatgaaaaac (tRNAGlu). The secondary structure of mt-tRNAMet was generated by tRNAscan (http://lowelab.ucsc.edu/tRNAscan-SE/).
Acknowledgments
We are grateful to A. Sharma for helping in the preparation of the FLAG-Ago2 construct, to G. Dreyfuss (University of Pennsylvania) for the myc-SMN and myc-hnRNPC1 constructs, to N. Doi and K. Saigo (University of Tokyo) for the Ago2-myc and Ago1-myc constructs, and to M. King and E. Davidson for illuminating discussions on mitochondrial genetics. This research was supported by grants from the NIH (R01-GM0720777) and the McCabe Foundation to Z.M.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2210805.
REFERENCES
- Aravin, A.A., Naumova, N.M., Tulin, A.V., Vagin, V.V., Rozovsky, Y.M., and Gvozdev, V.A. 2001. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11: 1017–1027. [DOI] [PubMed] [Google Scholar]
- Aravin, A.A., Klenov, M.S., Vagin, V.V., Bantignies, F., Cavalli, G., and Gvozdev, V.A. 2004. Dissection of a natural RNA silencing process in the Drosophila melanogaster germ line. Mol. Cell. Biol. 24: 6742–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell, M.A., Xuan, Z., Zhang, M.Q., and Hannon, G.J. 2002. The Argonaute family: Tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes & Dev. 16: 2733–2742. [DOI] [PubMed] [Google Scholar]
- Cikaluk, D.E., Tahbaz, N., Hendricks, L.C., DiMattia, G.E., Hansen, D., Pilgrim, D., and Hobman, T.C. 1999. GERp95, a membrane-associated protein that belongs to a family of proteins involved in stem cell differentiation. Mol. Biol. Cell 10: 3357–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, N., Zenno, S., Ueda, R., Ohki-Hamazaki, H., Ui-Tei, K., and Saigo, K. 2003. Short-interfering-RNA-mediated gene silencing in mammalian cells requires Dicer and eIF2C translation initiation factors. Curr. Biol. 13: 41–46. [DOI] [PubMed] [Google Scholar]
- Dounce, A.L., Witter, R.F., Monty, K.J., Pate, S., and Cottone, M.A. 1955. A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J. Biophys. Biochem. Cytol. 1: 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, A.N. and Macdonald, P.M. 2001. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development 128: 2823–2832. [DOI] [PubMed] [Google Scholar]
- Kobayashi, S., Amikura, R., and Okada, M. 1993. Presence of mitochondrial large ribosomal RNA outside mitochondria in germ plasm of Drosophila melanogaster. Science 260: 1521–1524. [DOI] [PubMed] [Google Scholar]
- Lippman, Z. and Martienssen, R. 2004. The role of RNA interference in heterochromatic silencing. Nature 431: 364–370. [DOI] [PubMed] [Google Scholar]
- Liu, J., Carmell, M.A., Rivas, F.V., Marsden, C.G., Thomson, J.M., Song, J.J., Hammond, S.M., Joshua-Tor, L., and Hannon, G.J. 2004. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441. [DOI] [PubMed] [Google Scholar]
- Meister, G. and Tuschl, T. 2004. Mechanisms of gene silencing by double-stranded RNA. Nature 431: 343–349. [DOI] [PubMed] [Google Scholar]
- Meister, G., Landthaler, M., Patkaniowska, A., Dorsett, Y., Teng, G., and Tuschl, T. 2004. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15: 185–197. [DOI] [PubMed] [Google Scholar]
- Mourelatos, Z., Dostie, J., Paushkin, S., Sharma, A., Charroux, B., Abel, L., Rappsilber, J., Mann, M., and Dreyfuss, G. 2002. miRNPs: A novel class of ribonucleoproteins containing numerous micro- RNAs. Genes & Dev. 16: 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison, E.P. and Hannon, G.J. 2004. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr. Opin. Cell Biol. 16: 223–229. [DOI] [PubMed] [Google Scholar]
- Nelson, P.T., Hatzigeorgiou, A.G., and Mourelatos, Z. 2004. miRNP:mRNA association in polyribosomes in a human neuronal cell line. RNA 10: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss, D., Josse, T., Anxolabehere, D., and Ronsseray, S. 2004. aubergine mutations in Drosophila melanogaster impair P cytotype determination by telomeric P elements inserted in heterochromatin. Mol. Genet. Genomics 272: 336–343. [DOI] [PubMed] [Google Scholar]
- Song, J.J., Smith, S.K., Hannon, G.J., and Joshua-Tor, L. 2004. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 305: 1434–1437. [DOI] [PubMed] [Google Scholar]
- Vastenhouw, N.L., Fischer, S.E., Robert, V.J., Thijssen, K.L., Fraser, A.G., Kamath, R.S., Ahringer, J., and Plasterk, R.H. 2003. A genome-wide screen identifies 27 genes involved in transposon silencing in C. elegans. Curr. Biol. 13: 1311–1316. [DOI] [PubMed] [Google Scholar]
- Wallace, D.C. 1999. Mitochondrial diseases in man and mouse. Science 283: 1482–1488. [DOI] [PubMed] [Google Scholar]