Abstract
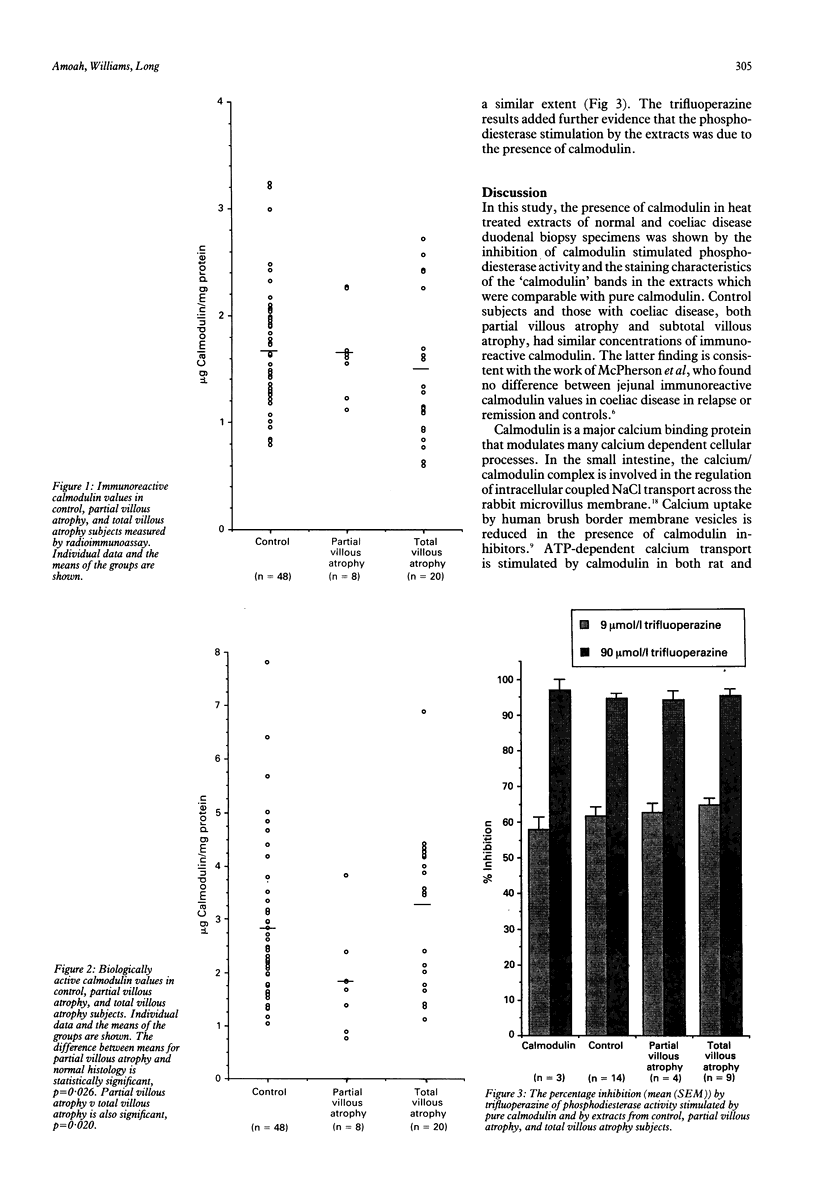
Calmodulin is an important modulator of intracellular calcium processes and may be implicated in the calcium malabsorption of coeliac disease. The calmodulin content in extracts of duodenal biopsy specimens from 48 normal control subjects and 28 patients with coeliac disease was determined. Radioimmunoassay was used to measure immunoreactive calmodulin while a cyclic adenosine 3',5'-monophosphate phosphodiesterase activity assay was used to measure biologically active calmodulin. Calmodulin values measured by both assays were similar for control and disease groups. Mean (SEM) immunoreactive calmodulin values were 1.68 (0.09) micrograms/mg protein for controls and 1.67 (0.15) and 1.45 (0.15) micrograms/mg protein for partial and total villous atrophy respectively. These values were not significantly different. Biologically active calmodulin values were 2.77 (0.21), 1.82 (0.34), and 3.24 (0.33) micrograms/mg protein for control, partial, and total villous atrophy subjects respectively. The biologically active calmodulin values in the partial villous atrophy group were significantly lower than in controls and total villous atrophy subjects. In the phosphodiesterase assay, the calmodulin antagonist trifluoperazine inhibited the activity stimulated by purified calmodulin and by the extracts to the same extent. These results show that calmodulin values are normal in coeliac disease and provide no evidence that changes in calmodulin account for the abnormal calcium absorption in these patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth C. C. Enterocyte in coeliac disease. 1. Br Med J. 1970 Sep 26;3(5725):725–731. doi: 10.1136/bmj.3.5725.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. C., Powell D. W. Calcium/calmodulin inhibition of coupled NaCl transport in membrane vesicles from rabbit ileal brush border. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5248–5252. doi: 10.1073/pnas.80.17.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghishan F. K., Arab N., Nylander W. Characterization of calcium uptake by brush border membrane vesicles of human small intestine. Gastroenterology. 1989 Jan;96(1):122–129. doi: 10.1016/0016-5085(89)90772-5. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Comparison of Ca++-regulated events in the intestinal brush border. J Cell Biol. 1985 Mar;100(3):754–763. doi: 10.1083/jcb.100.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Nagao S., Umeki S., Nozawa Y., Yazawa M., Yagi K. Ca2+-dependent modulator proteins from Tetrahymena pyriformis, sea anemone, and scallop and guanylate cyclase activation. J Biol Chem. 1981 Jan 10;256(1):19–22. [PubMed] [Google Scholar]
- Kikuchi K., Kikuchi T., Ghishan F. K. Characterization of calcium transport by basolateral membrane vesicles of human small intestine. Am J Physiol. 1988 Oct;255(4 Pt 1):G482–G489. doi: 10.1152/ajpgi.1988.255.4.G482. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Tucker W. F., Dawson R. A., Bleehen S. S., Tomlinson S. The calmodulin content of the epidermis in psoriasis. Clin Sci (Lond) 1985 Dec;69(6):681–688. doi: 10.1042/cs0690681. [DOI] [PubMed] [Google Scholar]
- Mac Neil S., Walker S. W., Brown B. L., Tomlinson S. Evidence that calmodulin may be involved in phytohaemagglutinin-stimulated lymphocyte division. Biosci Rep. 1982 Nov;2(11):891–897. doi: 10.1007/BF01114895. [DOI] [PubMed] [Google Scholar]
- MacNeil S., Griffin M., Cooke A. M., Pettett N. J., Dawson R. A., Owen R., Blackburn G. M. Calmodulin antagonists of improved potency and specificity for use in the study of calmodulin biochemistry. Biochem Pharmacol. 1988 May 1;37(9):1717–1723. doi: 10.1016/0006-2952(88)90434-0. [DOI] [PubMed] [Google Scholar]
- Macpherson A. J., Bjarnason I., Peters T. J. The subcellular distribution and levels of calmodulin in jejunal biopsies from control subjects and patients with coeliac disease. Clin Chim Acta. 1986 Sep 15;159(2):133–138. doi: 10.1016/0009-8981(86)90045-8. [DOI] [PubMed] [Google Scholar]
- Melvin K. E., Hepner G. W., Bordier P., Neale G., Joplin G. F. Calcium metabolism and bone pathology in adult coeliac disease. Q J Med. 1970 Jan;39(153):83–113. [PubMed] [Google Scholar]
- Rochette-Egly C., Lacroix B., Pflieger H., Doffoel M., Kedinger M., Haffen K. Calmodulin in normal and cystic fibrosis human intestine at different developmental stages. Gut. 1988 May;29(5):571–579. doi: 10.1136/gut.29.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shori D. K., Bradbury N. A., Goodchild M. C., Dormer R. L., McPherson M. A. An altered calmodulin binding protein in cystic fibrosis--a clue to the biochemical defect. Clin Chim Acta. 1988 Jun 15;174(3):283–289. doi: 10.1016/0009-8981(88)90054-x. [DOI] [PubMed] [Google Scholar]
- Staun M. Distribution of the 10,000 molecular weight calcium binding protein along the small and large intestine of man. Gut. 1987 Jul;28(7):878–882. doi: 10.1136/gut.28.7.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staun M., Jarnum S. Measurement of the 10,000-molecular weight calcium-binding protein in small-intestinal biopsy specimens from patients with malabsorption syndromes. Scand J Gastroenterol. 1988 Sep;23(7):827–832. doi: 10.3109/00365528809090768. [DOI] [PubMed] [Google Scholar]
- Stoll R., Stern H., Ruppin H., Domschke W. Effect of two potent calmodulin antagonists on calcium transport of brush border and basolateral vesicles from human duodenum. Aliment Pharmacol Ther. 1987 Oct;1(5):415–424. doi: 10.1111/j.1365-2036.1987.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Thomasset M., Molla A., Parkes O., Demaille J. G. Intestinal calmodulin and calcium-binding protein differ in their distribution and in the effect of vitamin D steroids on their concentration. FEBS Lett. 1981 May 5;127(1):13–16. doi: 10.1016/0014-5793(81)80329-8. [DOI] [PubMed] [Google Scholar]
- Tomlinson S., MacNeil S., Walker S. W., Ollis C. A., Merritt J. E., Brown B. L. Calmodulin and cell function. Clin Sci (Lond) 1984 May;66(5):497–507. doi: 10.1042/cs0660497. [DOI] [PubMed] [Google Scholar]
- Veigl M. L., Vanaman T. C., Sedwick W. D. Calcium and calmodulin in cell growth and transformation. Biochim Biophys Acta. 1984;738(1-2):21–48. doi: 10.1016/0304-419x(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. W., Tallant E. A., Cheung W. Y. Assay of calmodulin by Ca2+-dependent phosphodiesterase. Methods Enzymol. 1983;102:39–47. doi: 10.1016/s0076-6879(83)02006-6. [DOI] [PubMed] [Google Scholar]


