Abstract
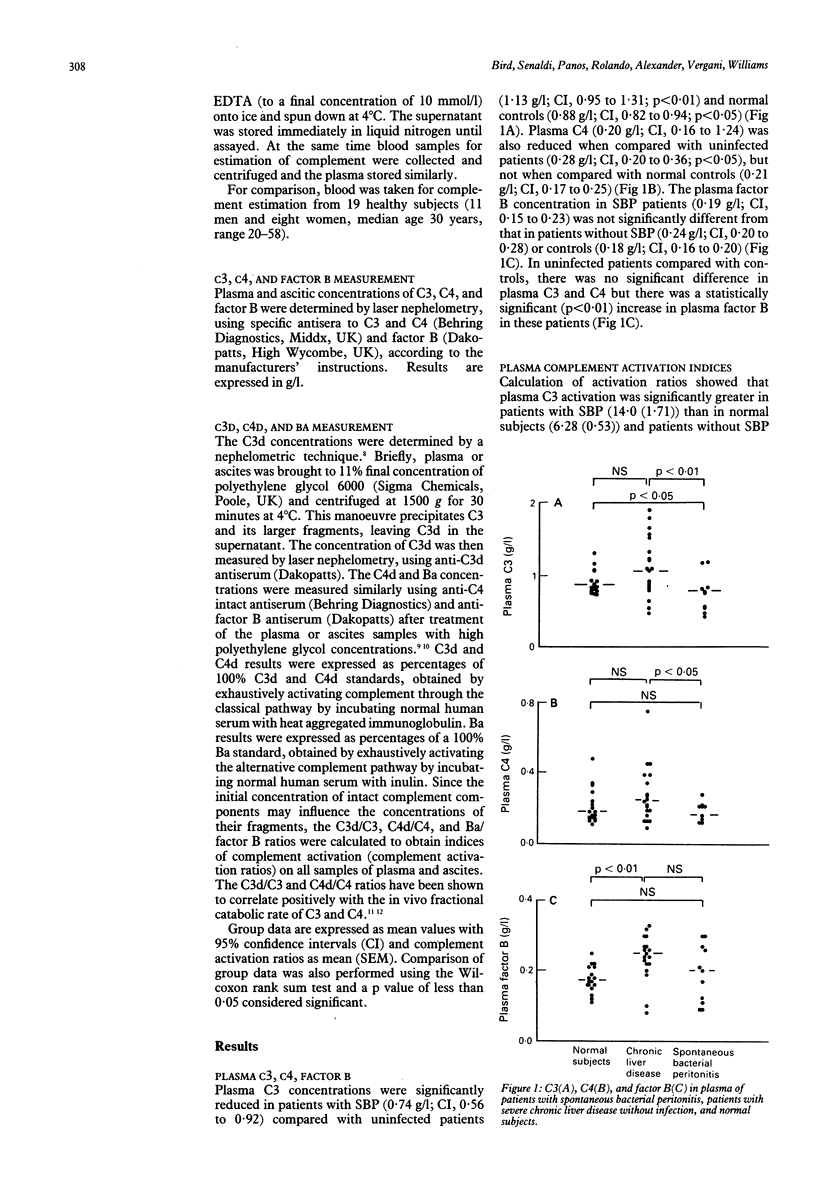
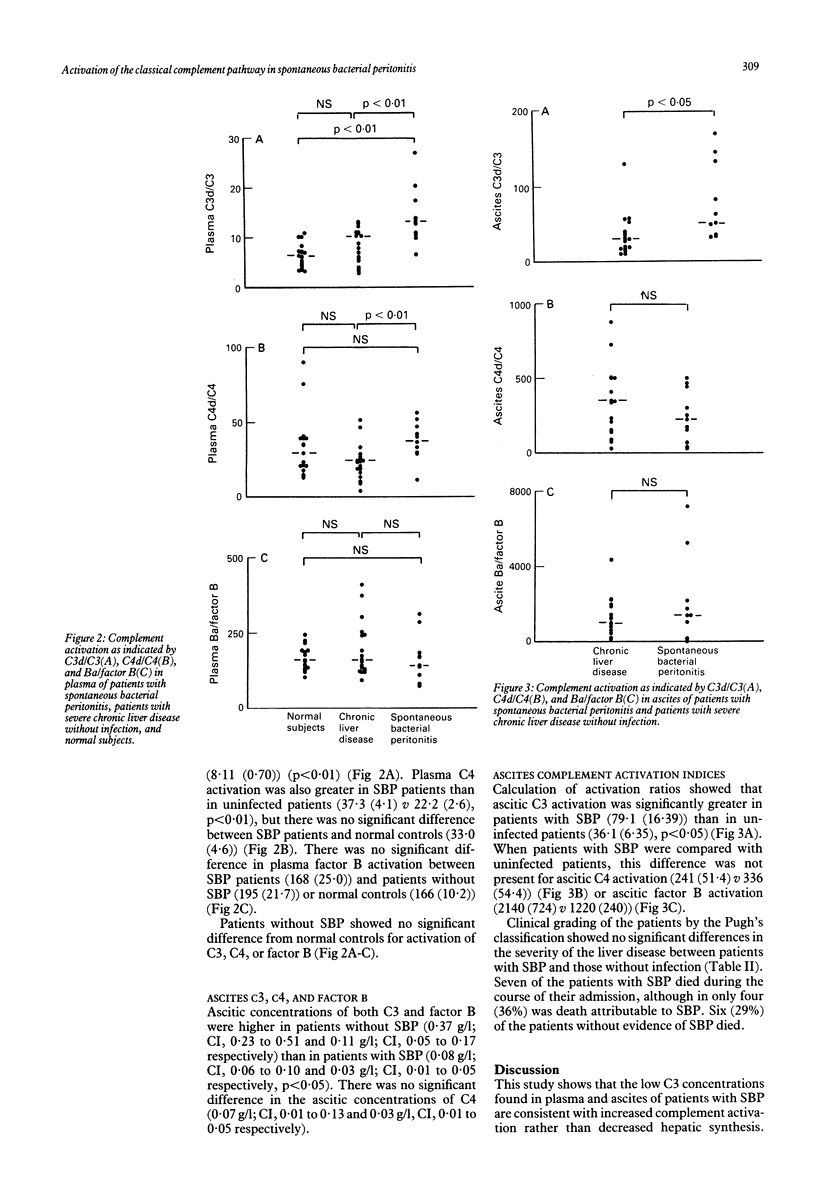
To investigate the possibility that low complement concentrations in the plasma and ascites of patients with severe liver disease could be secondary to complement consumption, complement activation was studied in 32 patients with severe liver disease, 11 of whom had spontaneous bacterial peritonitis (SBP). In patients with SBP, plasma C3 and C4 were significantly lower than in uninfected patients (mean values 0.74 v 1.13 g/l, p less than 0.01 and 0.20 v 0.28 g/l, p less than 0.05 respectively). Plasma complement activation via the classical pathway, as shown by C4d/C4, was significantly increased in patients with SBP compared with uninfected patients (37.3 v 22.2, p less than 0.01) as was C3d/C3 (14.0 v 8.11, p less than 0.01), but there was no significant difference in Ba/B between SBP and uninfected patients. Ascitic C3 concentrations were higher in patients without SBP than in infected patients (0.37 v 0.08 g/l, p less than 0.05), as were factor B values (0.11 v 0.03 g/l, p less than 0.05). There was no significant difference in ascitic C4 concentrations in patients with SBP compared with uninfected patients (0.03 v 0.07 g/l). Although consumption of C3, as shown by C3d/C3 in ascites, was increased in infected patients compared with uninfected patients (79.1 v 36.1, p less than 0.05), there was no difference in ascitic complement activation between the groups for either the classical or alternative pathways. In SBP, decreased plasma C3 and C4 are primarily caused by increased activation of the classical pathway and not impaired hepatic synthesis. Activation and consumption of C3 is one factor causing the low ascitic C3 concentrations observed in SBP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. E., McGivan J. D. Acetaldehyde alone may initiate hepatocellular damage in acute alcoholic liver disease. Gut. 1985 Oct;26(10):1065–1069. doi: 10.1136/gut.26.10.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E. T., Nasaruddin B. A., Alhaq A., Senaldi G., Vergani D. Clinical application of new technique that measures C4d for assessment of activation of classical complement pathway. J Clin Pathol. 1988 Feb;41(2):143–147. doi: 10.1136/jcp.41.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson N. D., Krohn K., Fauconnet M. H., Anderson K. E. Significance of serum complement levels in chronic liver disease. Gastroenterology. 1972 Oct;63(4):653–659. [PubMed] [Google Scholar]
- Fox R. A., Dudley F. J., Sherlock S. The serum concentration of the third component of complement beta-1C-beta-1A in liver disease. Gut. 1971 Jul;12(7):574–578. doi: 10.1136/gut.12.7.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourilsky O., Leroy C., Peltier A. P. Complement and liver cell function in 53 patients with liver disease. Am J Med. 1973 Dec;55(6):783–790. doi: 10.1016/0002-9343(73)90259-3. [DOI] [PubMed] [Google Scholar]
- Mal F., Huu T. P., Bendahou M., Trinchet J. C., Garnier M., Hakim J., Beaugrand M. Chemoattractant and opsonic activity in ascitic fluid. A study in 47 patients with cirrhosis or malignant peritonitis. J Hepatol. 1991 Jan;12(1):45–49. doi: 10.1016/0168-8278(91)90907-s. [DOI] [PubMed] [Google Scholar]
- Milgrom H., Curd J. G., Kaplan R. A., Müller-Eberhard H. J., Vaughan J. H. Activation of the fourth component of complement (C4): assessment by rocket immunoelectrophoresis and correlation with the metabolism of C4. J Immunol. 1980 Jun;124(6):2780–2785. [PubMed] [Google Scholar]
- Munoz L. E., De Villiers D., Markham D., Whaley K., Thomas H. C. Complement activation in chronic liver disease. Clin Exp Immunol. 1982 Mar;47(3):548–554. [PMC free article] [PubMed] [Google Scholar]
- Pinzello G., Simonetti R. G., Craxì A., Di Piazza S., Spanò C., Pagliaro L. Spontaneous bacterial peritonitis: a prospective investigation in predominantly nonalcoholic cirrhotic patients. Hepatology. 1983 Jul-Aug;3(4):545–549. doi: 10.1002/hep.1840030411. [DOI] [PubMed] [Google Scholar]
- Pugh R. N., Murray-Lyon I. M., Dawson J. L., Pietroni M. C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973 Aug;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Rajkovic I. A., Williams R. Mechanisms of abnormalities in host defences against bacterial infection in liver disease. Clin Sci (Lond) 1985 Mar;68(3):247–253. doi: 10.1042/cs0680247. [DOI] [PubMed] [Google Scholar]
- Runyon B. A., Antillon M. R., Montano A. A. Effect of diuresis versus therapeutic paracentesis on ascitic fluid opsonic activity and serum complement. Gastroenterology. 1989 Jul;97(1):158–162. doi: 10.1016/0016-5085(89)91430-3. [DOI] [PubMed] [Google Scholar]
- Runyon B. A., Canawati H. N., Akriviadis E. A. Optimization of ascitic fluid culture technique. Gastroenterology. 1988 Nov;95(5):1351–1355. doi: 10.1016/0016-5085(88)90372-1. [DOI] [PubMed] [Google Scholar]
- Runyon B. A. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology. 1988 May-Jun;8(3):632–635. doi: 10.1002/hep.1840080332. [DOI] [PubMed] [Google Scholar]
- Senaldi G., Peakman M., Alhaq A., Makinde V. A., Tee D. E., Vergani D. Activation of the alternative complement pathway: clinical application of a new technique to measure fragment Ba. J Clin Pathol. 1987 Oct;40(10):1235–1239. doi: 10.1136/jcp.40.10.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Such J., Guarner C., Enriquez J., Rodriguez J. L., Seres I., Vilardell F. Low C3 in cirrhotic ascites predisposes to spontaneous bacterial peritonitis. J Hepatol. 1988 Feb;6(1):80–84. doi: 10.1016/s0168-8278(88)80465-3. [DOI] [PubMed] [Google Scholar]
- Swaak A. J., van Rooyen A., Vogelaar C., Pillay M., Hack E. Complement (C3) metabolism in systemic lupus erythematosus in relation to the disease course. Rheumatol Int. 1986;6(5):221–226. doi: 10.1007/BF00541371. [DOI] [PubMed] [Google Scholar]
- Thompson R. A., Carter R., Stokes R. P., Geddes A. M., Goodall J. A. Serum immunoglobulins, complement component levels and autoantibodies in liver disease. Clin Exp Immunol. 1973 Jul;14(3):335–346. [PMC free article] [PubMed] [Google Scholar]
- Titó L., Rimola A., Ginès P., Llach J., Arroyo V., Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988 Jan-Feb;8(1):27–31. doi: 10.1002/hep.1840080107. [DOI] [PubMed] [Google Scholar]
- Vergani D., Bevis L., Nasaruddin B. A., Mieli-Vergani G., Tee D. E. Clinical application of a new nephelometric technique to measure complement activation. J Clin Pathol. 1983 Jul;36(7):793–797. doi: 10.1136/jcp.36.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Larsen G. L., Henson P. M. In vivo clearance and tissue distribution of C5a and C5a des arginine complement fragments in rabbits. J Clin Invest. 1982 Dec;70(6):1177–1183. doi: 10.1172/JCI110716. [DOI] [PMC free article] [PubMed] [Google Scholar]


