Abstract
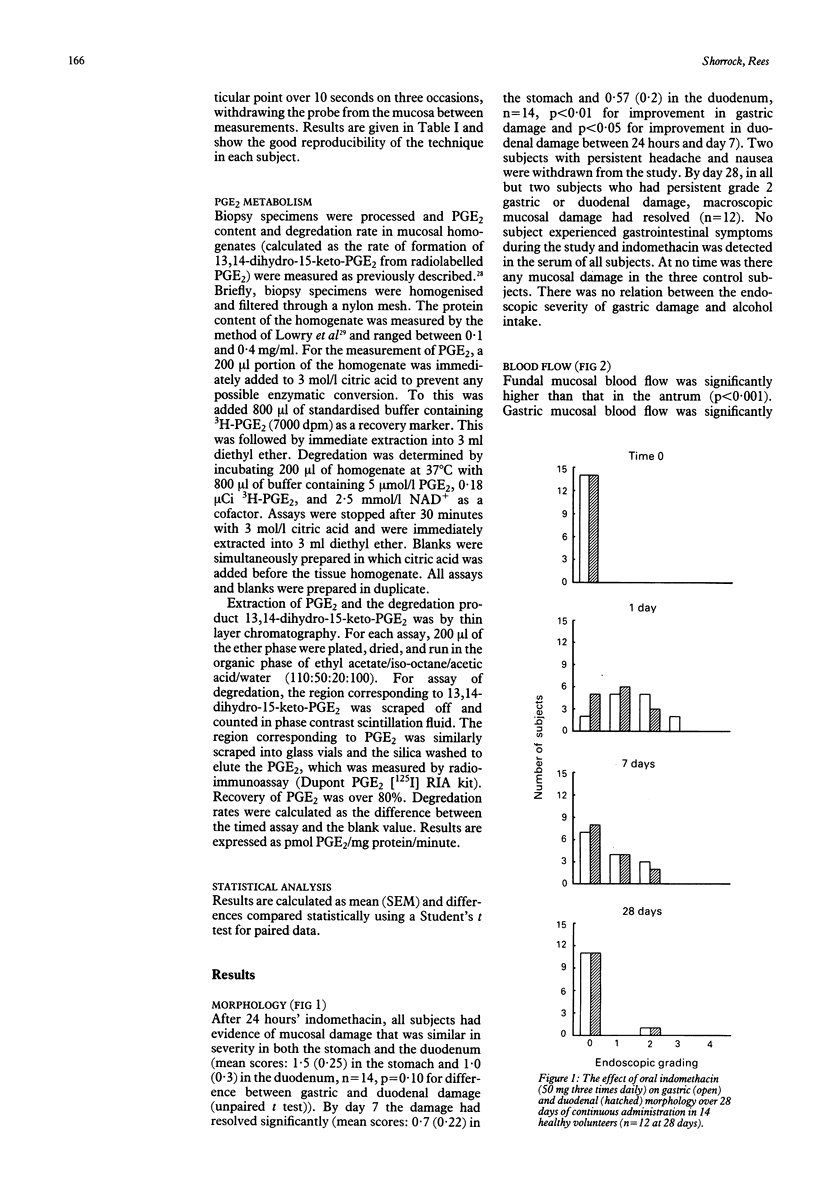
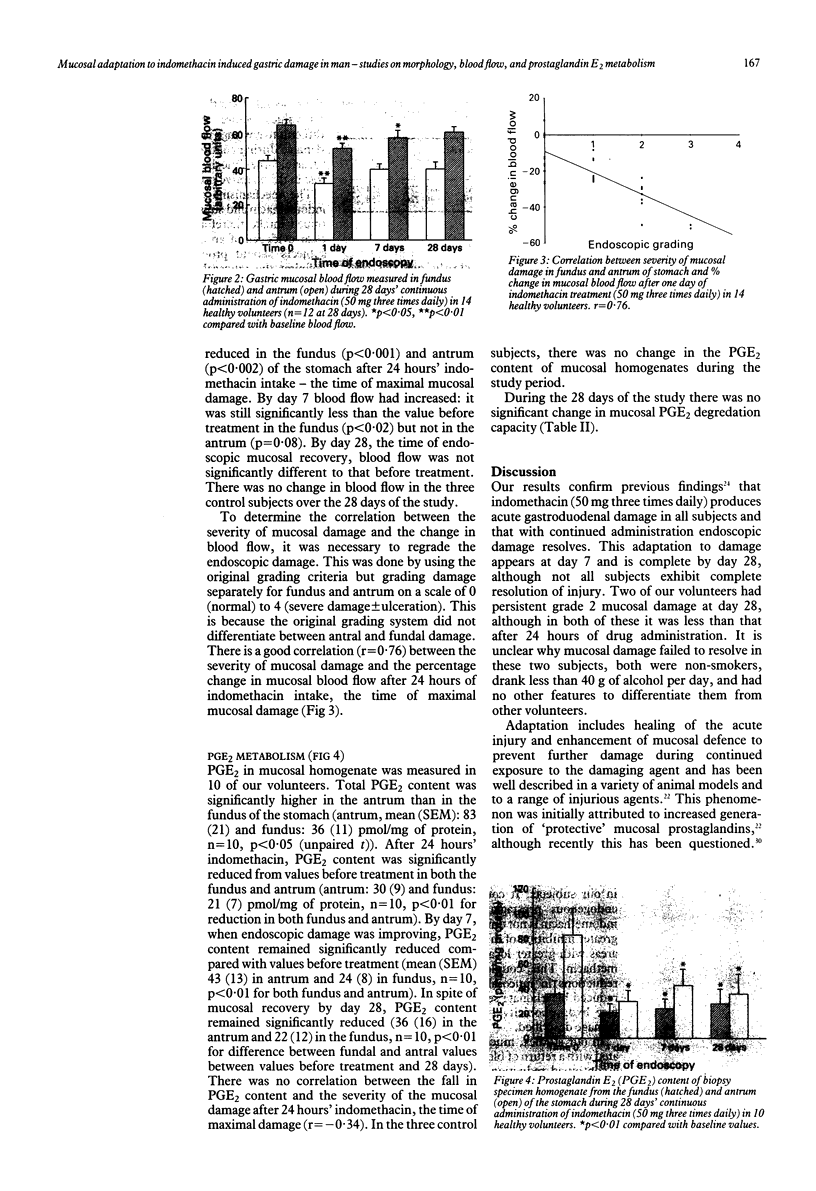
The effect of 28 days' continuous administration of oral indomethacin on gastroduodenal morphology, gastric mucosal blood flow, and gastric mucosal prostaglandin E2 (PGE2) metabolism in man was studied to define further the mechanisms of mucosal injury induced by indomethacin. Indomethacin caused acute gastroduodenal damage in all cases, which was maximal at 24 hours of administration. With continued intake, mucosal adaptation occurs resulting in resolution of endoscopic mucosal damage. At the time of maximal mucosal damage, gastric mucosal blood flow was significantly reduced compared with values before treatment (p less than 0.001 in fundus and p less than 0.002 in antrum), with good correlation between the severity of damage and the magnitude of the reduction in blood flow (r = 0.76). Mucosal recovery was associated with a return of the blood flow to normal. PGE2 in mucosal homogenate was significantly reduced by indomethacin in both the fundus (p less than 0.01) and antrum (p less than 0.01) after 24 hours but there was no correlation between the magnitude of this reduction and the severity of mucosal damage (r = -0.34). Despite mucosal recovery by 28 days, PGE2 values remained significantly below those before treatment in both the fundus (p less than 0.01) and antrum (p less than 0.01). The PGE2 degradation capacity was not influenced by indomethacin. In conclusion, mucosal adaptation to acute damage by indomethacin occurs in man and seems independent of local PGE2 metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. P., Blower A. L. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987 May;28(5):527–532. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley S. W., Sonnenschein L. A., Cheung L. Y. Focal gastric mucosal blood flow at the site of aspirin-induced ulceration. Am J Surg. 1985 Jan;149(1):53–59. doi: 10.1016/s0002-9610(85)80009-x. [DOI] [PubMed] [Google Scholar]
- Caruso I., Bianchi Porro G. Gastroscopic evaluation of anti-inflammatory agents. Br Med J. 1980 Jan 12;280(6207):75–78. doi: 10.1136/bmj.280.6207.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampton J. R., Gibbons L. C., Rees W. D. Simultaneous measurement of in vitro gastroduodenal prostaglandin E2 synthesis and degradation in peptic ulcer disease. Scand J Gastroenterol. 1987 May;22(4):425–430. doi: 10.3109/00365528708991485. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Spjut H. J., Torres E. Gastric adaptation. Studies in humans during continuous aspirin administration. Gastroenterology. 1988 Aug;95(2):327–333. [PubMed] [Google Scholar]
- Hawkey C. J., Kemp R. T., Walt R. P., Bhaskar N. K., Davies J., Filipowicz B. Evidence that adaptive cytoprotection in rats is not mediated by prostaglandins. Gastroenterology. 1988 Apr;94(4):948–954. doi: 10.1016/0016-5085(88)90552-5. [DOI] [PubMed] [Google Scholar]
- Kiel J. W., Riedel G. L., DiResta G. R., Shepherd A. P. Gastric mucosal blood flow measured by laser-Doppler velocimetry. Am J Physiol. 1985 Oct;249(4 Pt 1):G539–G545. doi: 10.1152/ajpgi.1985.249.4.G539. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Kwiecien N., Obtulowicz W., Kiec-Dembinska A., Polanski M., Kopp B., Sito E., Oleksy J. Effect of carprofen and indomethacin on gastric function, mucosal integrity and generation of prostaglandins in men. Hepatogastroenterology. 1982 Dec;29(6):267–270. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langman M. J. Epidemiologic evidence on the association between peptic ulceration and antiinflammatory drug use. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):640–646. doi: 10.1016/s0016-5085(89)80060-5. [DOI] [PubMed] [Google Scholar]
- Lanza F. L. Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. Am J Med. 1984 Jul 13;77(1A):19–24. doi: 10.1016/s0002-9343(84)80014-5. [DOI] [PubMed] [Google Scholar]
- Lanza F. L., Royer G. L., Jr, Nelson R. S., Chen T. T., Seckman C. E., Rack M. F. The effects of ibuprofen, indomethacin, aspirin, naproxen, and placebo on the gastric mucosa of normal volunteers: a gastroscopic and photographic study. Dig Dis Sci. 1979 Nov;24(11):823–828. doi: 10.1007/BF01324896. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Petokas S., Nandi J., Enthoven D. Effects of nonsteroidal, antiinflammatory drugs on gastrointestinal injury and prostanoid generation in healthy volunteers. Dig Dis Sci. 1988 Jun;33(6):660–666. doi: 10.1007/BF01540427. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M., Richards J. E., Hills B. A. Effect of 16,16-dimethyl prostaglandin E2 on the surface hydrophobicity of aspirin-treated canine gastric mucosa. Gastroenterology. 1985 Jan;88(1 Pt 2):308–314. doi: 10.1016/s0016-5085(85)80185-2. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Sestieri M., Karmeli F., Zimmerman J., Okon E., Rachmilewitz D. Rectal administration of nonsteroidal antiinflammatory drugs. Effect on rat gastric ulcerogenicity and prostaglandin E2 synthesis. Gastroenterology. 1990 May;98(5 Pt 1):1245–1249. [PubMed] [Google Scholar]
- MENGUY R., MASTERS Y. F. EFFECTS OF ASPIRIN ON GASTRIC MUCOUS SECRETION. Surg Gynecol Obstet. 1965 Jan;120:92–98. [PubMed] [Google Scholar]
- Main I. H., Whittle B. J. Investigation of the vasodilator and antisecretory role of prostaglandins in the rat gastric mucosa by use of non-steroidal anti-inflammatory drugs. Br J Pharmacol. 1975 Feb;53(2):217–224. doi: 10.1111/j.1476-5381.1975.tb07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainsford K. D. The effects of aspirin and other non-steroid anti-inflammatory/analgesic drugs on gastro-intestinal mucus glycoprotein biosynthesis in vivo: relationship to ulcerogenic actions. Biochem Pharmacol. 1978 Mar 15;27(6):877–885. doi: 10.1016/0006-2952(78)90412-4. [DOI] [PubMed] [Google Scholar]
- Redfern J. S., Lee E., Feldman M. Effect of indomethacin on gastric mucosal prostaglandins in humans. Correlation with mucosal damage. Gastroenterology. 1987 Apr;92(4):969–977. doi: 10.1016/0016-5085(87)90972-3. [DOI] [PubMed] [Google Scholar]
- Rees W. D., Gibbons L. C., Turnberg L. A. Effects of non-steroidal anti-inflammatory drugs and prostaglandins on alkali secretion by rabbit gastric fundus in vitro. Gut. 1983 Sep;24(9):784–789. doi: 10.1136/gut.24.9.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. D., Turnberg L. A. Reappraisal of the effects of aspirin on the stomach. Lancet. 1980 Aug 23;2(8191):410–413. doi: 10.1016/s0140-6736(80)90452-3. [DOI] [PubMed] [Google Scholar]
- Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979 Oct;77(4 Pt 1):761–767. [PubMed] [Google Scholar]
- Shepherd A. P., Riedel G. L. Continuous measurement of intestinal mucosal blood flow by laser-Doppler velocimetry. Am J Physiol. 1982 Jun;242(6):G668–G672. doi: 10.1152/ajpgi.1982.242.6.G668. [DOI] [PubMed] [Google Scholar]
- Shorrock C. J., Prescott R. J., Rees W. D. The effects of indomethacin on gastroduodenal morphology and mucosal pH gradient in the healthy human stomach. Gastroenterology. 1990 Aug;99(2):334–339. doi: 10.1016/0016-5085(90)91013-v. [DOI] [PubMed] [Google Scholar]
- Somerville K., Faulkner G., Langman M. Non-steroidal anti-inflammatory drugs and bleeding peptic ulcer. Lancet. 1986 Mar 1;1(8479):462–464. doi: 10.1016/s0140-6736(86)92927-2. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., McKnight G. W. The mucoid cap over superficial gastric damage in the rat. A high-pH microenvironment dissipated by nonsteroidal antiinflammatory drugs and endothelin. Gastroenterology. 1990 Aug;99(2):295–304. doi: 10.1016/0016-5085(90)91009-u. [DOI] [PubMed] [Google Scholar]
- Walt R., Katschinski B., Logan R., Ashley J., Langman M. Rising frequency of ulcer perforation in elderly people in the United Kingdom. Lancet. 1986 Mar 1;1(8479):489–492. doi: 10.1016/s0140-6736(86)92940-5. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Higgs G. A., Eakins K. E., Moncada S., Vane J. R. Selective inhibition of prostaglandin production in inflammatory exudates and gastric mucosa. Nature. 1980 Mar 20;284(5753):271–273. doi: 10.1038/284271a0. [DOI] [PubMed] [Google Scholar]


