Abstract
Drusen are extracellular deposits that accumulate below the retinal pigment epithelium on Bruch's membrane and are risk factors for developing age-related macular degeneration (AMD). The progression of AMD might be slowed or halted if the formation of drusen could be modulated. To work toward a molecular understanding of drusen formation, we have developed a method for isolating microgram quantities of drusen and Bruch's membrane for proteome analysis. Liquid chromatography tandem MS analyses of drusen preparations from 18 normal donors and five AMD donors identified 129 proteins. Immunocytochemical studies have thus far localized ≈16% of these proteins in drusen. Tissue metalloproteinase inhibitor 3, clusterin, vitronectin, and serum albumin were the most common proteins observed in normal donor drusen whereas crystallin was detected more frequently in AMD donor drusen. Up to 65% of the proteins identified were found in drusen from both AMD and normal donors. However, oxidative protein modifications were also observed, including apparent crosslinked species of tissue metalloproteinase inhibitor 3 and vitronectin, and carboxyethyl pyrrole protein adducts. Carboxyethyl pyrrole adducts are uniquely generated from the oxidation of docosahexaenoate-containing lipids. By Western analysis they were found to be more abundant in AMD than in normal Bruch's membrane and were found associated with drusen proteins. Carboxymethyl lysine, another oxidative modification, was also detected in drusen. These data strongly support the hypothesis that oxidative injury contributes to the pathogenesis of AMD and suggest that oxidative protein modifications may have a critical role in drusen formation.
Age-related macular degeneration (AMD) is the most common cause of legal blindness in the elderly population of developed countries, constituting a growing health problem (reviewed in ref. 1). Between 6 million and 10 million Americans are blind from AMD, and hundreds of thousands of new cases are diagnosed in the U.S. each year. Macular degeneration is characterized by the breakdown of the macula, the small portion of the central retina (≈2 mm in diameter) responsible for high-acuity vision. Genetic studies have shown that identical twins exhibit 100% concordance for AMD (2) and that gene mutations cause early-onset forms of macular degeneration (3–7). Late-onset macular degeneration (i.e., AMD) is usually defined as either “dry” or “wet” and is a slow, progressive disease with genetic influences as well as environmental risk factors such as cigarette smoking and perhaps diet and lifetime light exposure (1). The wet, exudative, neovascular form of AMD affects only ≈10% of those with the disease and is characterized by abnormal blood vessels growing from the choriocapillaris through the retinal pigment epithelium (RPE), typically resulting in hemorrhage, exudation, scarring, and/or serous retinal detachment (8). Approximately 90% of patients with AMD have the non-neovascular dry form characterized by atrophy of the RPE and loss of macular photoreceptors (8). At present there is no cure for any form of AMD, although some success in attenuating choroidal neovascularization has been obtained with photodynamic therapy (8).
The causal events responsible for AMD are not known. With age, debris-like material, referred to as drusen, accumulates below the RPE on Bruch's membrane. Drusen can be observed during a funduscopic eye examination, and these deposits are referred to as either soft drusen or hard drusen, clinical terms defining their relative size, abundance, and shape (reviewed in refs. 8 and 9). Normal eyes may have maculas free of drusen, yet drusen may be abundant in the retinal periphery (10). The presence of numerous and/or confluent, soft drusen in the macula is considered a major risk factor for developing AMD. This correlation is so well established that many clinicians refer to individuals with soft drusen in the macula, in the absence of any loss of macular vision, as an early stage of AMD (8, 11). Although drusen are widely accepted as contributors to the etiology of AMD, their composition and the mechanism of their formation is not understood. Histochemical and immunocytochemical studies have shown that drusen contain a variety of lipids, polysaccharides, and glycosaminoglycans (8, 9) and have identified ≈20 drusen proteins (12–14). Better definition of the molecular composition of drusen should provide important clues regarding drusen biogenesis as well as possible leads for drug targets and therapeutic agents to prevent AMD. We report here direct proteomic analysis of drusen isolated from normal and AMD donor eyes and present evidence of oxidative protein modifications that may be linked to drusen formation and underlie the pathogenesis of AMD.
Methods
Human Tissue Procurement.
Eyes from 29 normal human donors and 16 AMD donors, all between 56 and 98 years of age, were used in this study, including 23 eyes (18 normal, five AMD) used for drusen isolation and protein identification and 22 eyes (11 normal, 11 AMD) used for Western analyses. Normal eyes were obtained through the Cleveland Eye Bank or the National Disease Research Interchange (Philadelphia). AMD donor eyes were from clinically diagnosed patients registered through the Eye Donor Program of the Foundation Fighting Blindness (Owings Mills, MD). Eyes were enucleated between 1.5 and 7 h after death and frozen in liquid nitrogen. Tissues obtained from sources outside Cleveland were transferred to the laboratory on dry ice, and then stored at −80°C until used.
Protein Identification.
Lipids were extracted from isolated drusen preparations with chloroform/methanol (15). After lipid extraction, the aqueous fraction was dried and dissolved in 5 μl of 400 mM ammonium bicarbonate containing 8 M urea. Cysteine was reduced by adding 1 μl of 100 mM DTT, and after 30 min at room temperature under argon, 1 μl of 200 mM iodoacetamide was added and the alkylation continued 30 min under the same conditions. The preparation was then diluted 3-fold with water, incubated with trypsin (100 ng) overnight at 37°C, and centrifuged to remove insoluble material by using established methods (16). Soluble tryptic peptides were analyzed by liquid chromatography tandem MS (LC MS/MS) with a CapLC system (Micromass, Beverly, MA) and a quadrupole time-of-flight mass spectrometer (QTOF2, Micromass) as described (17). Peptides were separated on a 75 μm × 5 cm Biobasic C18 column (New Objective, Cambridge, MA) by using aqueous formic acid/acetonitrile solvents, a flow rate of 250 nl/min, and a gradient of 5–40% acetonitrile over 120 min followed by 80% acetonitrile for 5 min. Protein identifications from MS/MS data used proteinlynx global server and MASSLYNX 3.5 software (Micromass) and the Swiss-Protein and National Center for Biotechnology Information protein sequence databases. Identification of proteins by peptide mass mapping using in-gel tryptic digestion and a Voyager DE Pro matrix-assisted laser desorption ionization-time-of-flight mass spectrometer (Perkin–Elmer) was as described (18, 19).
Immunocytochemistry.
Immunocytochemical analyses to confirm drusen localization of proteins identified by MS were performed with commercially available antibodies by using methods as described (12, 20). Monoclonal antibodies to annexin II and VI were obtained from BD Biosciences, San Jose, CA, and used at 5–10 μg/ml; rabbit polyclonal antibodies to annexin I were purchased from Zymed and used at 25 μg/ml. Goat polyclonal antibodies to calgranulin A and B were obtained from Santa Cruz Biotechnology and used at 2 μg/ml; sheep polyclonal antibodies to psoriasin were from Fitzgerald Industries International, Concord, MA, and used at 10 μg/ml. Monoclonal antibody to carboxymethyllysine was purchased from Wako Chemical, Richmond, VA, and used at 5 μg/ml. Monoclonal anti-carboxyethyl pyrrole (CEP) antibodies to CEP adducts were prepared in the Hybridoma Core Facility, Lerner Research Institute, Cleveland Clinic Foundation and used at 5 μg/ml (21).
Western Analysis.
For 1D or 2D Western analysis, Bruch's membrane/RPE choroid was dissected from frozen AMD and normal eyes as described (12). Tissues were homogenized in 7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 0.5% Triton X-100, 2% ampholytes (pH 3–10), and 1% DTT, and subjected to either 1D or 2D electrophoresis. Electrophoresis and Western blotting methods were as described (17, 18). Rabbit polyclonal anti-CEP antibodies that recognize CEP adducts (21) were used as a purified IgG fraction (at 1 μg/ml) in Western analyses of dissected Bruch's membrane/RPE choroid (20 μg per lane).
Results
Drusen Isolation.
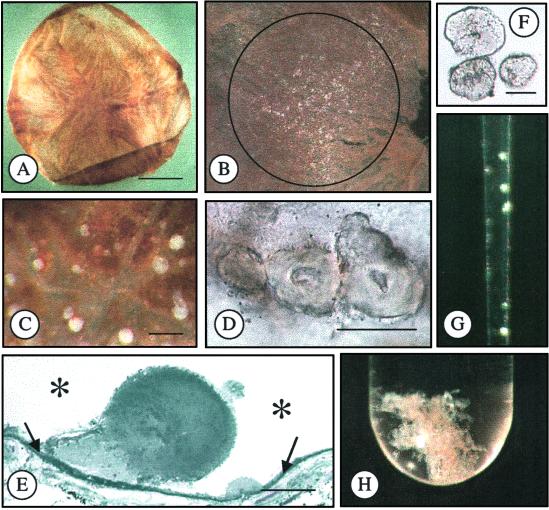
Eyes used for drusen isolation were thawed overnight at 4°C, the anterior segment was removed with a circumferential cut behind the limbus, the vitreous was poured from the posterior globe, the optic nerve was cut, and the retina was removed. Drusen appear as bump-like elevations of the RPE when viewed at magnifications of 20–40 diameters and the macular region of the RPE is identifiable by its heavier pigmentation. The RPE was removed from the interior surface of the globe under a dissecting microscope with repetitive brushing and aspiration in the presence of PBS. At this stage, drusen appear as opaque, 50- to 250-μm spherical to irregular deposits and remain attached to Bruch's membrane. To facilitate isolation, the choroid-Bruch's membrane complex was removed from the globe (Fig. 1), the region below the macula was isolated with a 6-mm trephine, and the remainder was cut into smaller fragments that lay flat in a Petri dish. At magnifications between 25 and 40 diameters, drusen were removed with fine forceps. Many of the drusen isolated were from far peripheral locations, known to be an abundant source of drusen in normal eyes (10). Smaller drusen (≤20 μm) were observed by removing most of the choroid, leaving only the choriocapillaris/Bruch's membrane complex. When these preparations were illuminated horizontally, small drusen could be easily identified on the surface of Bruch's membrane. In tissues with heavy drusen deposits, the material could be scraped from Bruch's membrane with blunt forceps or a small blade. These procedures allow drusen with distinct morphological features to be identified, sorted into separate groupings, and transferred to storage tubes with a capillary pipette for further analysis (Fig. 1). Based on Coomassie blue staining after SDS/PAGE of the most abundant drusen sample isolated, the average protein yield was in the low microgram range (<10 μg).
Fig. 1.
Isolation of drusen. (A) Bruch's membrane/choroid dissected from an 82-year-old donor eye. (B) Macular region of Bruch's membrane containing drusen (white particulate material), diameter = 3 mm. (C) Drusen on surface of Bruch's membrane. (D) Drusen on Bruch's membrane. (E) Histological section of drusen on Bruch's membrane (embedded in plastic, stained with toluidine blue). Asterisks represent the location of the RPE before removal and the arrows indicate Bruch's membrane. (F) Isolated drusen. (G) Isolated drusen in pipette. (H) Isolated drusen in test tube. (Bars = A, 5 mm; C, 100 μm; D, 50 μm; E and F, 25 μm.)
Drusen Proteins.
We have identified 129 proteins by LC MS/MS analyses of individual tryptic digests of isolated drusen preparations (see Table 3, which is published on the PNAS web site, www.pnas.org). These identifications include overlapping sets of 107 proteins from 18 different normal donors and 62 proteins from five different AMD donors. The number of proteins identified per drusen preparation varied between 0 and 39, depending on the quantity of protein available and performance of the LC MS/MS instrumentation, and averaged 19 proteins per drusen preparation in Table 3. The mass spectrometric analyses did not measure the abundance of the proteins identified. However, based on observed frequency among the donors analyzed, the most common proteins found in drusen from normal donors and AMD donors are listed in Table 1. Tissue metalloproteinase inhibitor 3 (TIMP3), clusterin, vitronectin and serum albumin appear to be common drusen proteins, detectable in up to 80% of the tissues from the 18 normal donors (Table 1) and 60% of drusen preparations from the five AMD donors (Table 3). Crystallins were detected more frequently in AMD donor drusen (Table 1); however, analysis of additional AMD donors is required to establish the significance of this observation.
Table 1.
Most common proteins in drusen
| Most common protein
|
Accession no.
|
Normal donors | AMD donors | ||
|---|---|---|---|---|---|
| Match | No. | Match | No. | ||
| Normal donor drusen | |||||
| Clusterin | P10909 | 10 | 16 | 5 | 4 |
| TIMP3 | P35625 | 14 | 16 | 6 | 3 |
| Serum albumin | P02768 | 10 | 11 | 27 | 3 |
| Vitronectin | P04004 | 8 | 11 | 7 | 3 |
| Complement component 9 | P02748 | 9 | 10 | 5 | 3 |
| Actin, beta | 5 | 8 | 5 | 2 | |
| Annexin II | NP_004030 | 3 | 8 | 12 | 2 |
| Histone H2B C | Q99880 | 5 | 7 | 1 | 2 |
| Lactoglobulin, beta A chain | P02754 | 5 | 7 | 3 | 5 |
| Apolipoprotein E | 4 | 6 | 2 | 2 | |
| Complement component 3 | P01024 | 6 | 6 | ||
| Complement component 8 | P07358 | 2 | 6 | 1 | 1 |
| Histone H2Ao | P20670 | 3 | 6 | 4 | 1 |
| Serum amyloid P | 1SACA | 3 | 6 | ||
| AMBP protein | P02760 | 6 | 5 | 1 | 1 |
| Histone H2A2 | P28001 | 2 | 5 | 3 | 3 |
| Novel leucine-rich protein | 7 | 5 | |||
| Vimentin | P08670 | 4 | 5 | 4 | 2 |
| AMD donor drusen | |||||
| Crystallin, beta B1 | P53674 | 7 | 2 | 15 | 5 |
| Lactoglobulin, beta A chain | P02754 | 5 | 7 | 3 | 5 |
| Clusterin | P10909 | 10 | 16 | 5 | 4 |
| Complement component 9 | P02748 | 9 | 10 | 5 | 3 |
| Crystallin, alpha B | P02511 | 5 | 1 | 7 | 3 |
| Crystallin, beta A3 | P05813 | 4 | 1 | 8 | 3 |
| Crystallin, beta A4 | P53673 | 2 | 1 | 4 | 3 |
| Crystallin, beta B2 | P43320 | 2 | 1 | 9 | 3 |
| Crystallin, beta S | P22914 | 2 | 2 | 8 | 3 |
| Hemoglobin beta 2 | P02023 | 4 | 3 | 5 | 3 |
| Histone H2A2 | P28001 | 2 | 5 | 3 | 3 |
| Serum albumin | P02768 | 10 | 11 | 27 | 3 |
| TIMP3 | P35625 | 14 | 16 | 6 | 3 |
| Vitronectin | P04004 | 8 | 11 | 7 | 3 |
The age and sex of normal donors (n = 18) was 87F, 87M, 88F, 90F, 91F1, 91F2, 91F3, 92F1, 92F2, 92F3, 93M1, 93M2, 93M3, 94F, 96F1, 96F2, 98F1, 98F2. The age and sex of AMD donors (n = 5) was 56F, 63F, 93M, 95M, 96F. Match indicates maximum number of peptide matches identified per analysis by LC MS/MS sequence analysis. No. indicates the number of donors exhibiting the indicated protein.
Swiss-Protein database accession nos. are shown in regular text and National Center for Biotechnology Information database accession nos. are in italics.
Immunocytochemical Localization of Drusen Proteins.
Of the 129 proteins identified by LC MS/MS analysis (Table 3), we have obtained immunocytochemical evidence supporting the existence of the following proteins in drusen: annexins I and VI, calgranulin A and B, psoriasin (Figs. 4 and 5, which are published as supporting information on the PNAS web site), clusterin (20), and TIMP3 (12). Annexin II was identified by LC MS/MS, but immunocytochemical analyses localized this protein to the basal plasma membrane of the RPE adhering to drusen (Fig. 4). Previously published immunocytochemical studies have identified several other proteins in drusen that we also encountered by LC MS/MS, including vitronectin (13), amyloid A, amyloid P, α1-antitrypsin, α1-antichymotrypsin, apolipoprotein A1, apolipoprotein E, complement components C3, C5, and C9, fibrinogen, Ig kappa, Ig lambda, and ubiquitin (14).
Drusen Oxidative Protein Modifications.
A particularly abundant drusen preparation from a normal donor was subjected to SDS/PAGE, consecutive 1-mm slices were excised throughout the gel lane, and each was subjected to in situ tryptic digestion followed by capillary LC MS/MS analysis (Fig. 2 and Table 2). Proteins identified were the more common components of normal donor drusen, including TIMP3 and vitronectin, which migrated in multiple mass ranges, from the top to the bottom of the gel (Fig. 2; Table 2). The multiple, higher mass electrophoretic components containing these proteins suggest the presence of covalent crosslinks, protein modifications that can be caused by reactive lipid and carbohydrate oxidation products (22, 23).
Fig. 2.
SDS/PAGE analysis of drusen. Drusen isolated from a 93-year-old male, normal donor was dissolved in Laemmli sample buffer and ≈10 μg was fractionated by SDS/PAGE (4% stacking, 7.5% separating polyacrylamide gel). The gel was stained with Coomassie blue, bands were excised, and proteins were identified by LC MS/MS as described in Methods.
Table 2.
SDS/PAGE analysis of drusen
| Protein | Gel band | Gel observed mass, kDa | Calculated mass | Accession no. | Peptide matches |
|---|---|---|---|---|---|
| Vitronectin | 1, 9, 10, 11, 14–20, 22, 25 | 220 < gel top to dye front < 25 | 54287 | P04004 | 7 |
| Metalloproteinase inhibitor-3 | 1, 12–15, 17, 19, 20, 22–25 | 220 < gel top to dye front < 25 | 24127 | P35625 | 4 |
| Complement C9 | 1 | >220 | 63155 | P02748 | 4 |
| Lysozyme C | 7 | 100 | 14560 | P81709 | 1 |
| Clusterin | 17–20, 22, 23 | 25–40 | 52476 | P10909 | 9 |
| Serum amyloid P | 20, 21, 22 | 25–35 | 25369 | P02743 | 7 |
| Serum albumin | 10 | 80 | 69348 | P02768 | 4 |
| Apolipoprotein E | 19, 20 | 30–40 | 36136 | P02649 | 3 |
Drusen isolated from a 93-year-old male, normal donor was dissolved in Laemmli sample buffer and ≈10 μg was fractionated by SDS/PAGE (4% stacking, 7.5% separating polyacrylamide gel). The gel was stained with Coomassie blue, bands were excised, and proteins were identified by LC MS/MS as described in Methods.
Band excised from the gel in Fig. 2.
Sequence calculated mass.
Swiss-Protein database accession no.
Maximum no. of peptide sequence matches detected.
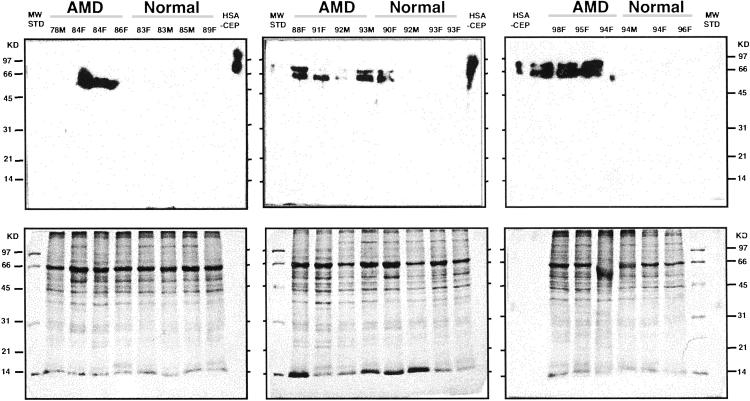
Immunocytochemical analysis of Bruch's membrane/choroid tissue from a normal donor demonstrated CEP and carboxymethyllysine immunoreactivity in drusen and Bruch's membrane (Fig. 6, which is published as supporting information on the PNAS web site). CEP protein modifications are uniquely generated by oxidation products of docosahexaenoate-containing lipids (21) and carboxymethyllysine is one of many advanced glycation end products (AGEs) generated by oxidation of carbohydrate (24). In complementary experiments, Bruch's membrane/RPE/choroid from AMD and age- and gender-matched normal tissue donors were screened by Western analysis for CEP modifications. Western results (Fig. 3) revealed more frequent CEP immunoreactivity among the AMD samples than in the normal controls. Specifically, nine of the 11 AMD samples were immunopositive whereas only two of the 11 age-matched controls were CEP immunoreactive. Statistically these CEP immunoreactivity results are significantly different between AMD and normal donors (Fischer's test, P value = 0.004 single-sided).
Fig. 3.
Western analysis of AMD and normal Bruch's membrane/RPE/choroid tissues. Human Bruch's membrane/RPE/choroid from the macular region of AMD and normal donor eyes was subjected to SDS/PAGE (≈20 μg protein/lane), electroblotted to poly(vinylidene difluoride), and probed with the rabbit polyclonal anti-CEP antibody to CEP adducts from docosahexaenoic acid. Human serum albumin modified with CEP (HSA-CEP, 20 ng) was used as a positive control. The age and sex of the donor eyes are listed at the top of each lane. More immunoreactivity can be seen in the AMD samples than in the normals.
In subsequent 2D gel Western analyses of Bruch's membrane/ RPE/choroid from an AMD donor (84-year-old female), several CEP immunoreactive proteins were identified by matrix-assisted laser desorption ionization-time-of-flight MS (Fig. 7, which is published as supporting information on the PNAS web site). Among the nine identified immunopositive proteins were albumin and α1-antitrypsin, both of which were detected in drusen by LC MS/MS (Table 3) and immunocytochemistry (14). Also identified with apparent CEP immunoreactivity (Fig. 7) was the X-linked juvenile retinoschisis protein (XLRS). Mutations in the XLRS gene cause retinal degeneration (25); oxidative modification of XLRS may be another mechanism of pathogenesis and/or play a role in the pathogenesis of AMD.
Discussion
Drusen are a hallmark risk factor for developing AMD, yet little is known about their molecular composition or mechanism of formation. The progression of AMD might be slowed or halted if the formation of drusen could be modulated. As an approach to better understanding the molecular mechanisms of drusen formation, we have initiated direct analysis of the proteins in drusen. A step toward this goal has been the development of a method for isolating microgram quantities of drusen for mass spectrometric analysis. We have now performed drusen dissections on more than 60 human eyes, including the 23 eyes used in this study for drusen protein identification, and including 13 donors for which both the right and left eyes were dissected. The following general conclusions regarding the isolation procedure can be made: (i) Most drusen remain on Bruch's membrane after brushing to remove the RPE. (ii) The abundance of drusen varies per donor and accordingly, the amount of protein recovered is variable and in the low microgram range. (iii) Not every drusen preparation has yielded protein identifications by LC MS/MS. Our observations during drusen isolation are consistent with previous funduscopic reports indicating (i) that most elderly eyes have drusen, particularly in the periphery; (ii) when a large population of drusen are found in one eye, the other eye also contains abundant drusen; and (iii) multiple drusen types (hard and soft) can be present in a single eye (8–10).
LC MS/MS analysis of the isolated drusen preparations after tryptic digestion has led to the identification of a cumulative total of 129 proteins. Although we expect the majority of these are components of drusen, the possibility exists that some may be soluble contaminating proteins or as in the case of annexin II, localized to RPE tissue adhering to drusen. To verify drusen localization, immunocytochemical studies are ongoing, and to date 21 of the identified proteins (16%) have been confirmed as drusen components in our laboratories and elsewhere.
Heterogeneity as well as protein compositional similarities were apparent among the analyzed drusen preparations. About 38% (41/107) of the proteins identified from normal donor drusen were also found in drusen from AMD donors and 65% (42/62) of the proteins identified from AMD donor drusen were found in tissues from normal donors. Twenty-one of the 62 proteins detected in drusen from AMD tissue (≈33%) were not observed in normal donor drusen (Table 3). None of the identified proteins suggest an obvious pathway or mechanism for drusen formation, and whether any drusen protein is uniquely associated with AMD remains to be determined. However, the detection of a hypothetical protein in AMD drusen leaves open the possibility that specific proteins may become useful therapeutic drug targets as we learn more.
Cumulative oxidative damage contributes to aging and has long been suspected of contributing to the pathogenesis of AMD (26). Perhaps the most compelling evidence that oxidative damage plays a role in AMD are epidemiological studies showing that smoking significantly increases the risk of AMD (1, 27), and that for select individuals, the progression of the disease can be slowed with antioxidant vitamins and zinc (28). Protein modifications generated from lipoxidation and glyoxidation include a variety of crosslinks, and we have observed apparent crosslinked species of the common drusen proteins TIMP3 and vitronectin. Furthermore, our Western analysis of Bruch's membrane preparations suggest that docosahexaenoate lipid-derived oxidative modifications (i.e., CEP-protein adducts) are more abundant in AMD than normal tissues. By matrix-assisted laser desorption ionization-time-of-flight MS analysis of AMD Bruch's membrane after 2D gel Western analysis, we have also found CEP immunoreactivity associated with albumin and α1-antitrypsin, two confirmed drusen proteins. Notably, docosahexaenoate is abundant in photoreceptor cell outer segments and is the most oxidizable fatty acid in humans (29). In addition to our observations regarding AGEs (ref. 30; Fig. 6), protein modifications from oxidized carbohydrate (e.g., carboxymethyllysine and pentosidine) have been reported in ocular tissues from aged donors and those with AMD (31, 32). Owing to the high photooxidative stress in the retina, other oxidative modifications are likely to be found in drusen such as tyrosine nitration, which appears to be modulated by light in rat retina (17).
Prevailing hypotheses regarding the origin of drusen are that drusen components are either derived from the RPE or the choroidal vasculature (9, 33). The proteins we have identified are found in several tissues, including the RPE, blood, and photoreceptors, and accordingly, support both the RPE and the choroidal vasculature as sources of the components in drusen. The observation of plasma proteins in drusen that are up-regulated during an inflammatory response has led to the mechanistic proposal that immune-mediated, complement activation events, triggered by signals from the RPE, are causally involved in the formation of drusen (9, 34). Others have associated immune-mediated events with the pathogenesis of AMD (33), but it is not yet clear whether this influence is a causative or a secondary contributory event. Oxidative protein modifications such as protein crosslinks, AGEs, and lipid derived modifications may be the primary catalysts in drusen formation. Indeed AGEs stimulate vascular endothelial growth factor expression (35) and angiogenesis in vivo (36) and may play a role in choroidal neovascularization in AMD. A plausible drusen biogenesis scenario might first involve proteins like TIMP3, clusterin, and vitronectin, which normally interact with sulfated glycosaminoglycans (GAGs) (37–39), to noncovalently bind to the GAGs in Bruch's membrane. As oxidative stress defense mechanisms deteriorate with age, oxidative modifications may gradually lock these and other proteins to Bruch's membrane with crosslinks, preventing normal turnover and initiating drusen development. The photooxidative environment in the retina and the lipid-rich photoreceptor outer segments, 10% of which are phagocytosed daily by the RPE, provide an excellent source of reactive oxygen species. Waste products from the RPE and blood components from the choriocapillaris provide a ready source of extracellular material for oxidative modification and drusen formation. Over time, oxidative modifications and subsequent immune-mediated events could cause the expansion of drusen on Bruch's membrane. Accordingly, we hypothesize that oxidative protein modifications are causally involved in drusen formation.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants EY06603, EY02362, GM21249, EY014239, and EY014240, a Research Center grant from The Foundation Fighting Blindness, a grant from Merck, Inc., and funds from the Wolf Family Foundation and The Cleveland Clinic Foundation.
Abbreviations
AGE, advanced glycation end product
AMD, age-related macular degeneration
CEP, carboxyethyl pyrrole
LC MS/MS, liquid chromatography tandem MS
RPE, retinal pigment epithelium
TIMP3, tissue metalloproteinase inhibitor 3
See commentary on page 14619.
References
- 1.Evans J. R. (2001) Prog. Retinal Eye Res. 20, 227-253. [DOI] [PubMed] [Google Scholar]
- 2.Meyers S. M. & Zachary, A. A. (1988) Arch. Ophthalmol. 106, 651-653. [DOI] [PubMed] [Google Scholar]
- 3.Allikmets R., Singh, N., Sun, H., Shroyer, N. F., Hutchinson, A., Chidambaram, A., Gerard, B., Baird, L., Stauffer, D., Peiffer, A., et al. (1997) Nat. Genet. 15, 236-245. [DOI] [PubMed] [Google Scholar]
- 4.Zhang K., Kniazeva, M., Han, M., Li, W., Yu, Z., Yang, Z., Li, Y., Metzker, M. L., Allikmets, R., Zack, D. J., et al. (2001) Nat. Genet. 27, 89-93. [DOI] [PubMed] [Google Scholar]
- 5.Petrukhin K., Koisti, M. J., Bakall, B., Li, W., Xie, G., Marknell, T., Sandgren, O., Forsman, K., Holmgren, G., Andreasson, S., et al. (1998) Nat. Genet. 19, 241-247. [DOI] [PubMed] [Google Scholar]
- 6.Felbor U., Stohr, H., Amann, T., Schonherr, U. & Weber, B. H. (1995) Hum. Mol. Genet. 4, 2415-2416. [DOI] [PubMed] [Google Scholar]
- 7.Stone E. M., Lotery, A. J., Munier, F. L., Heon, E., Piguet, B., Guymer, R. H., Vandenburgh, K., Cousin, P., Nishimura, D., Swiderski, R. E., et al. (1999) Nat. Genet. 22, 199-202. [DOI] [PubMed] [Google Scholar]
- 8.Abdelsalam A., Del Priore, L. & Zarbin, M. A. (1999) Surv. Ophthalmol. 44, 1-28. [DOI] [PubMed] [Google Scholar]
- 9.Hageman G. S., Luthert, P. J., Chong, N. H. V., Johnson, L. V., Anderson, D. H. & Mullins, R. F. (2001) Prog. Retinal Eye Res. 20, 705-732. [DOI] [PubMed] [Google Scholar]
- 10.Lewis H. B., Straatsma, B. R. & Foos, R. Y. (1986) Ophthalmology 93, 1098-1111. [DOI] [PubMed] [Google Scholar]
- 11.Midena E., Deglii Angeli, C., Blarzino, M., Valenti, M. & Segato, T. (1997) Invest. Ophthalmol. Visual Sci. 38, 469-477. [PubMed] [Google Scholar]
- 12.Kamei M. & Hollyfield, J. E. (1999) Invest. Ophthalmol. Visual Sci. 40, 2367-2375. [PubMed] [Google Scholar]
- 13.Hageman G. S, Mullins, R. F., Russell, S. R., Johnson, L. V. & Anderson, D. H. (1999) FASEB J. 13, 477-484. [DOI] [PubMed] [Google Scholar]
- 14.Mullins R. F., Russell, S. R., Anderson, D. H. & Hageman, G. S. (2000) FASEB J. 14, 835-846. [PubMed] [Google Scholar]
- 15.Bligh E. G. & Dyer, W. J. (1959) Can. J. Biochem. Physiol. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- 16.Crabb J. W., Nie, Z., Chen, Y., Hulmes, J. D., West, K. A., Kapron, J. T., Ruuska, S. E., Noy, N. & Saari, J. C. (1998) J. Biol. Chem. 273, 20712-20720. [DOI] [PubMed] [Google Scholar]
- 17.Miyagi M., Sakaguchi, H., Darrow, R. M., Yan, L., West, K. A., Aulak, K. S., Stuehr, D. J., Hollyfield, J. G., Organisciak, D. T. & Crabb, J. W. (2002) Mol. Cell. Proteomics 1, 293-303. [DOI] [PubMed] [Google Scholar]
- 18.West K. A., Yan, L., Miyagi, M., Crabb, J. S., Marmorstein, A. D., Marmorstein, L. & Crabb, J. W. (2001) Exp. Eye Res. 73, 479-491. [DOI] [PubMed] [Google Scholar]
- 19.Aulak K. S., Masaru, M., Yan, L., West, K. A., Massillon, D., Crabb, J. W. & Stuehr, D. J. (2001) Proc. Natl. Acad. Sci. USA 98, 12056-12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi H., Miyagi, M., Shadrach, K. G., Rayborn, M. E., Crabb, J. W. & Hollyfield, J. G. (2002) Exp. Eye Res. 74, 547-549. [DOI] [PubMed] [Google Scholar]
- 21.Hollyfield, J. G., Salomon, R. G., Crabb, J. W. & Gu, X. (2002) U.S. Patent Application 10/135,196.
- 22.Friguet B., Stadtman, E. R. & Szweda, L. I. (1994) J. Biol. Chem. 269, 21639-21643. [PubMed] [Google Scholar]
- 23.Elgawish A., Glomb, M., Friedlander, M. & Monnier, V. M. R. (1996) J. Biol. Chem. 271, 12964-12971. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed M. U., Thorpe, S. R. & Baynes, J. W. (1986) J. Biol. Chem. 261, 4889-4894. [PubMed] [Google Scholar]
- 25.Sauer C. G., Gehrig, A., Warneke-Wittstock, R., Marquardt, A., Ewing, C. C., Gibson, A., Lorenz, B., Jurklies, B. & Weber, B. H. F. (1997) Nat. Genet. 17, 164-170. [DOI] [PubMed] [Google Scholar]
- 26.Beatty S., Koh, H., Phil, M., Henson, D. & Boulton, M. (2000) Surv. Ophthalmol. 45, 115-134. [DOI] [PubMed] [Google Scholar]
- 27.Seddon J. M., Willett, W. C., Speizer, F. E. & Hankinson, S. E. (1996) J. Am. Med. Assoc. 276, 1141-1146. [PubMed] [Google Scholar]
- 28.AREDS Research Group (2001) Arch. Ophthalmol. 119, 1417-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fliesler S. J. & Anderson, R. E. (1983) Prog. Lipid Res. 22, 79-131. [DOI] [PubMed] [Google Scholar]
- 30.Call T. W. & Hollyfield, J. G. (1990) Exp. Eye Res. 51, 451-462. [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi T., Murata, T., Hangai, M., Nagai, R., Horiuchi, S., Lopez, P. F., Hinton, D. R. & Ryan, S. J. (1998) Arch. Ophthalmol. 116, 1629-1632. [DOI] [PubMed] [Google Scholar]
- 32.Handa J. T., Verzijl, N., Matsunaga, H., Aotaki-Keen, A., Lutty, G. A., te Koppele, J. M., Miyata, T. & Hjelmeland, L. M. (1999) Invest. Ophthalmol. Visual Sci. 40, 775-779. [PubMed] [Google Scholar]
- 33.Penfold P. L., Madigan, M. C., Gilles, M. C. & Provis, J. M. (2001) Prog. Retinal Eye Res. 20, 385-414. [DOI] [PubMed] [Google Scholar]
- 34.Johnson L. V., Ozaki, S., Staples, M. L., Erickson, P. A. & Anderson, D. H. (2000) Exp. Eye Res. 70, 441-449. [DOI] [PubMed] [Google Scholar]
- 35.Treins C., Giorgetti-Peraldi, S., Murdaca, J. & Van Obberghen, E. (2001) J. Biol. Chem. 276, 43836-43841. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto T., Tanaka, S., Stan, A. C., Koike, T., Kase, M., Makita, Z., Sawa, E. & Nagashima, K. (2002) Microvasc. Res. 63, 186-195. [DOI] [PubMed] [Google Scholar]
- 37.Yu W. H., Yu, S., Meng, Q., Brew, K. & Woessner, J. F., Jr. (2000) J. Biol. Chem. 275, 31226-31232. [DOI] [PubMed] [Google Scholar]
- 38.Pankhurst G. J., Bennett, C. A. & Easterbrook-Smith, S. B. (1998) Biochemistry 37, 4823-4830. [DOI] [PubMed] [Google Scholar]
- 39.Hogasen K., Mollnes, T. E. & Harboe, M. (1992) J. Biol. Chem. 267, 23076-23082. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.