Abstract
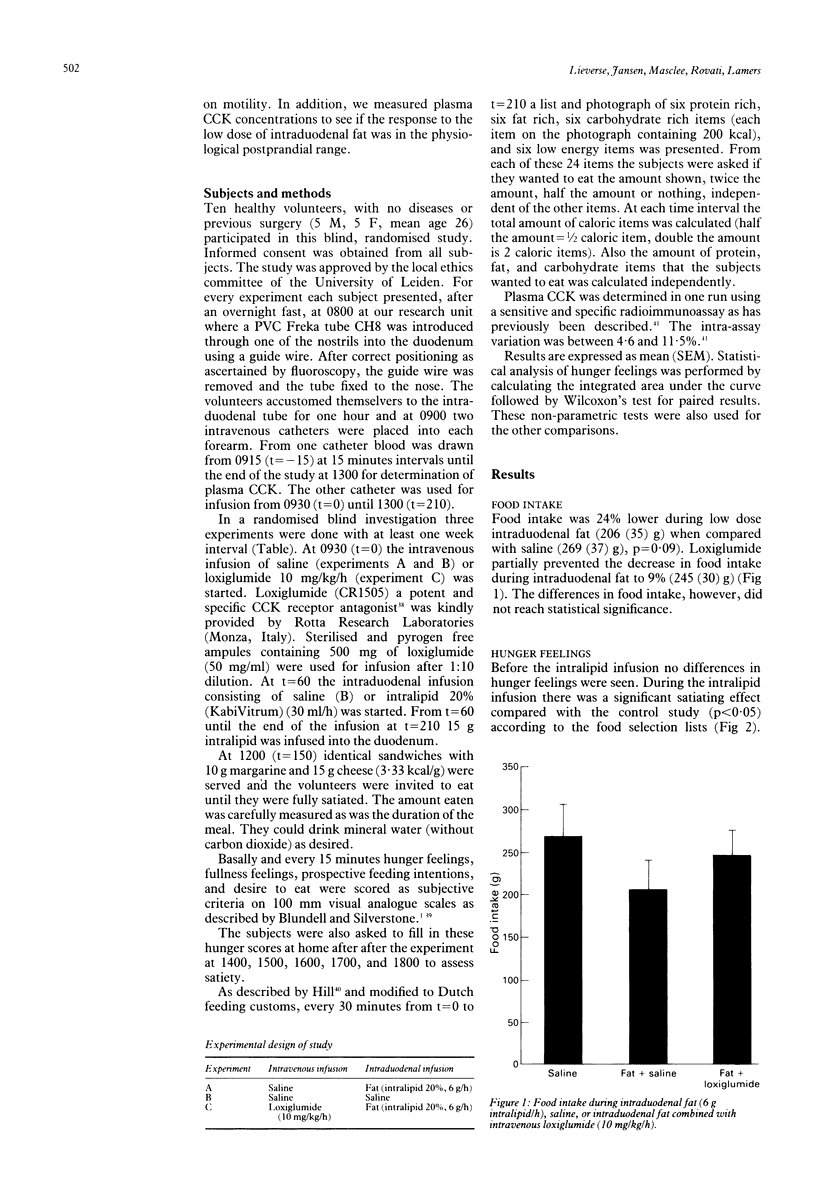
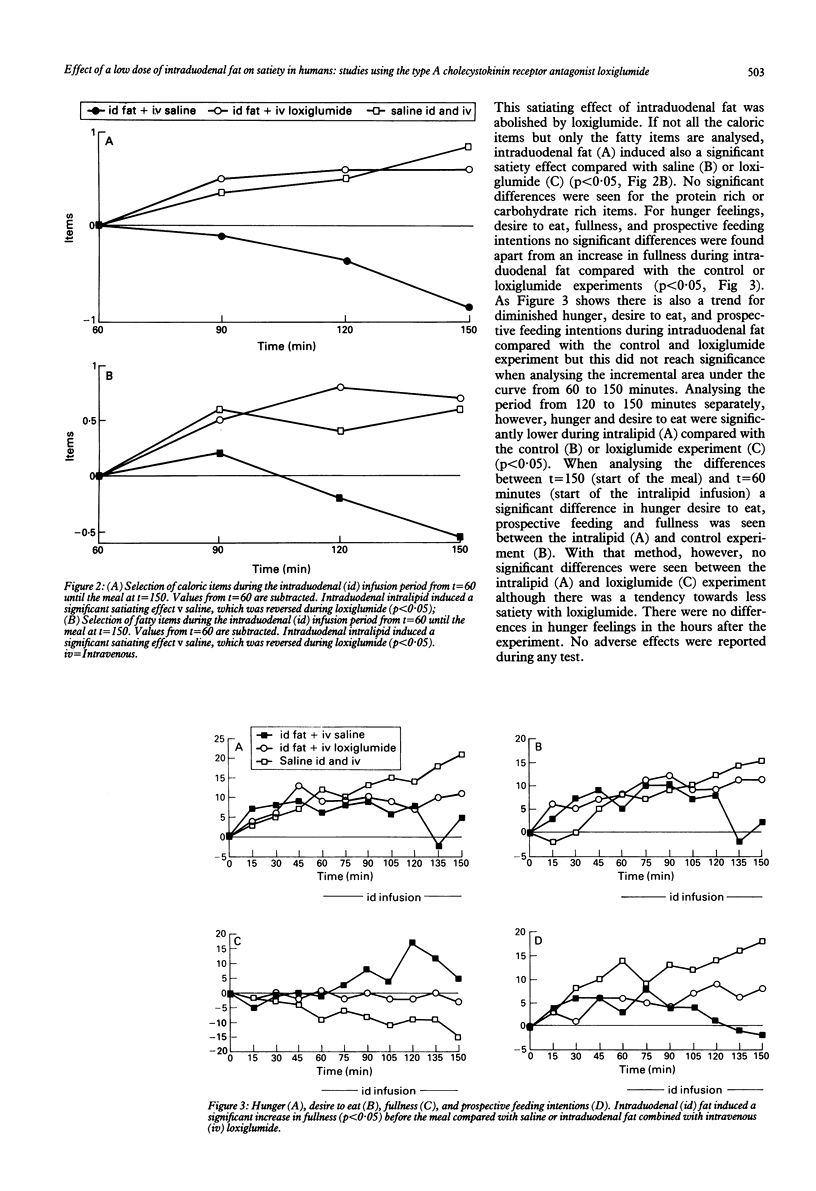
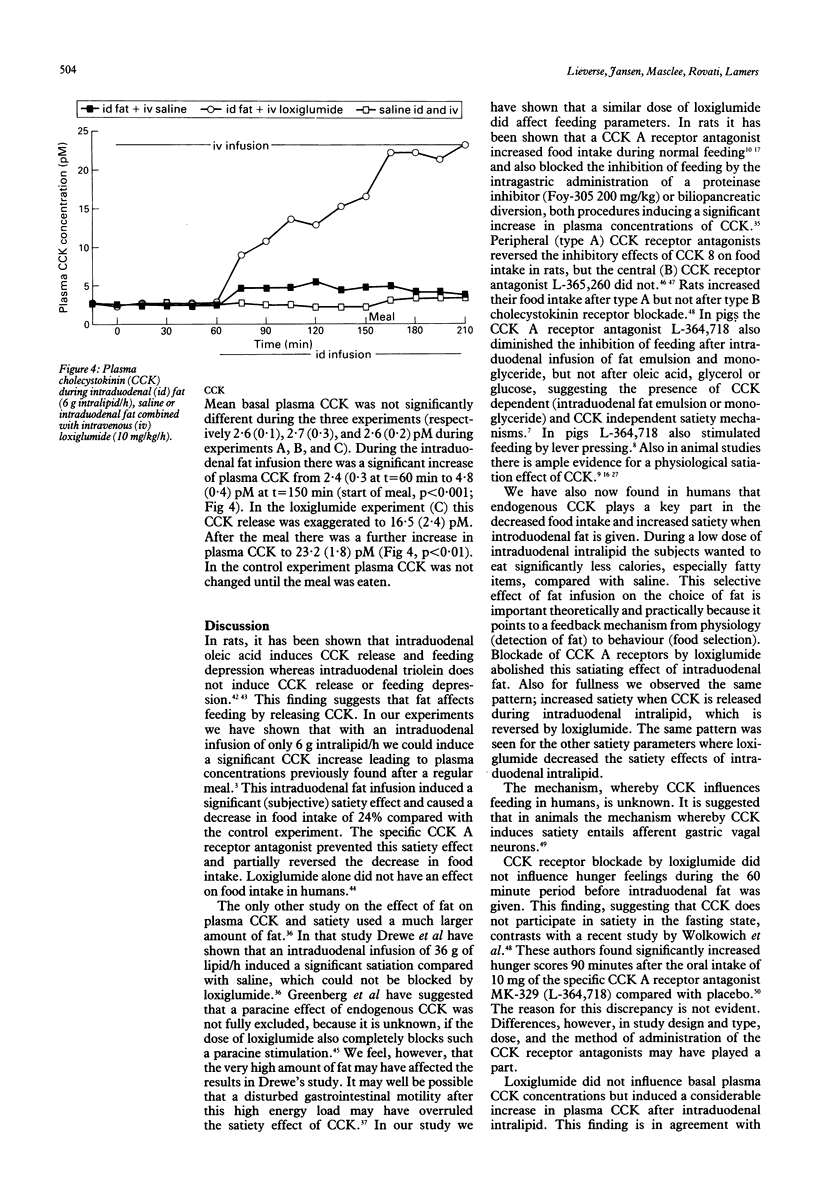
Satiation, the process that brings eating to an end, and satiety, the state of inhibition over further eating, may be influenced by cholecystokinin (CCK). In animal and human studies, it has been shown that infusion of exogenous CCK decreases food intake, but the doses given may well have led to supraphysiological plasma concentrations. This study was done to discover if a low dose of intraduodenal fat releasing physiological amounts of endogenous cholecystokinin exerts satiation or satiety effects, or both and if these effects could be inhibited by the CCK receptor antagonist loxiglumide. In 10 healthy lean volunteers (5 F, 5 M, mean age 26) three tests were performed in a randomised blind fashion. Intralipid 20% (6 g/h) (experiments A and C) or saline (experiment B) were given intraduodenally from 1030 until 1300. The subjects received saline (experiments A and B) or loxiglumide (experiment C) a specific CCK-receptor antagonist (10 mg/kg/h) intravenously from 0930 until 1300. At 1200 a meal was served. At regular time intervals hunger feelings were measured using visual analogue scales and food selection lists and plasma CCK was measured by radioimmunoassay. Food intake (mean (SEM)) during intraduodenal fat (206(35)g) was lower than in the control study (269(37)g, p = 0.09). Loxiglumide largely prevented the inhibitory effect of intraduodenal fat on food intake (245(30)g). From 1030 until the meal at 1200 there was a significant satiating effect of intraduodenal fat compared with the control and loxiglumide experiments according to the food selection lists, which was because of the satiating effect for the fat rich items (p<0.05). Also feelings of fullness were significantly higher during intraduodenal fat than in the control or loxiglumide experiments (p<0.05). During intraduodenal fat there was a significant increase of plasma CCK from 2.4(0.3) to 4.8(0.4) pM (p<0.001). Loxiglumide led to an exaggerated CCK release to a peak concentration of 16(2.4) pM before the meal. This study shows that in humans low dose intraduodenal fat increases satiety and satiation, mainly through the effect of CCK.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bado A., Rodriguez M., Lewin M. J., Martinez J., Dubrasquet M. Cholecystokinin suppresses food intake in cats: structure-activity characterization. Pharmacol Biochem Behav. 1988 Oct;31(2):297–303. doi: 10.1016/0091-3057(88)90349-8. [DOI] [PubMed] [Google Scholar]
- Baile C. A., McLaughlin C. L., Della-Fera M. A. Role of cholecystokinin and opioid peptides in control of food intake. Physiol Rev. 1986 Jan;66(1):172–234. doi: 10.1152/physrev.1986.66.1.172. [DOI] [PubMed] [Google Scholar]
- Blundell J. E., Burley V. J. Satiation, satiety and the action of fibre on food intake. Int J Obes. 1987;11 (Suppl 1):9–25. [PubMed] [Google Scholar]
- Blundell J. Pharmacological approaches to appetite suppression. Trends Pharmacol Sci. 1991 Apr;12(4):147–157. doi: 10.1016/0165-6147(91)90532-w. [DOI] [PubMed] [Google Scholar]
- Corwin R. L., Gibbs J., Smith G. P. Increased food intake after type A but not type B cholecystokinin receptor blockade. Physiol Behav. 1991 Jul;50(1):255–258. doi: 10.1016/0031-9384(91)90529-w. [DOI] [PubMed] [Google Scholar]
- Dourish C. T., Ruckert A. C., Tattersall F. D., Iversen S. D. Evidence that decreased feeding induced by systemic injection of cholecystokinin is mediated by CCK-A receptors. Eur J Pharmacol. 1989 Dec 7;173(2-3):233–234. doi: 10.1016/0014-2999(89)90528-1. [DOI] [PubMed] [Google Scholar]
- Drewe J., Gadient A., Rovati L. C., Beglinger C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology. 1992 May;102(5):1654–1659. doi: 10.1016/0016-5085(92)91726-k. [DOI] [PubMed] [Google Scholar]
- Ebenezer I. S., de la Riva C., Baldwin B. A. Effects of the CCK receptor antagonist MK-329 on food intake in pigs. Physiol Behav. 1990 Jan;47(1):145–148. doi: 10.1016/0031-9384(90)90053-7. [DOI] [PubMed] [Google Scholar]
- Figlewicz D. P., Sipols A. J., Porte D., Jr, Woods S. C., Liddle R. A. Intraventricular CCK inhibits food intake and gastric emptying in baboons. Am J Physiol. 1989 Jun;256(6 Pt 2):R1313–R1317. doi: 10.1152/ajpregu.1989.256.6.R1313. [DOI] [PubMed] [Google Scholar]
- Garlicki J., Konturek P. K., Majka J., Kwiecien N., Konturek S. J. Cholecystokinin receptors and vagal nerves in control of food intake in rats. Am J Physiol. 1990 Jan;258(1 Pt 1):E40–E45. doi: 10.1152/ajpendo.1990.258.1.E40. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Young R. C., Smith G. P. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973 Sep;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Gourch A., Orosco M., Rodriguez M., Martinez J., Cohen Y., Jacquot C. Effects of a new cholecystokinin analogue (JMV 236) on food intake and brain monoamines in the rat. Neuropeptides. 1990 Jan;15(1):37–41. doi: 10.1016/0143-4179(90)90158-u. [DOI] [PubMed] [Google Scholar]
- Greenberg D., Smith G. P., Gibbs J. Cholecystokinin and the satiating effect of fat. Gastroenterology. 1992 May;102(5):1801–1803. doi: 10.1016/0016-5085(92)91746-q. [DOI] [PubMed] [Google Scholar]
- Gregory P. C., McFadyen M., Rayner D. V. Duodenal infusion of fat, cholecystokinin secretion and satiety in the pig. Physiol Behav. 1989 May;45(5):1021–1024. doi: 10.1016/0031-9384(89)90232-1. [DOI] [PubMed] [Google Scholar]
- Hewson G., Leighton G. E., Hill R. G., Hughes J. The cholecystokinin receptor antagonist L364,718 increases food intake in the rat by attenuation of the action of endogenous cholecystokinin. Br J Pharmacol. 1988 Jan;93(1):79–84. doi: 10.1111/j.1476-5381.1988.tb11407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman W. P., Jansen J. B., Lamers C. B. Comparative study of the effects of equal amounts of fat, protein, and starch on plasma cholecystokinin in man. Scand J Gastroenterol. 1985 Sep;20(7):843–847. doi: 10.3109/00365528509088832. [DOI] [PubMed] [Google Scholar]
- Jansen J. B., Jebbink M. C., Douglas B. R., Lamers C. B. Effect of loxiglumide (CR-1505) on bombesin- and meal-stimulated plasma cholecystokinin in man. Eur J Clin Pharmacol. 1990;38(4):367–370. doi: 10.1007/BF00315577. [DOI] [PubMed] [Google Scholar]
- Jansen J. B., Lamers C. B. Radioimmunoassay of cholecystokinin in human tissue and plasma. Clin Chim Acta. 1983 Jul 15;131(3):305–316. doi: 10.1016/0009-8981(83)90100-6. [DOI] [PubMed] [Google Scholar]
- Kissileff H. R., Pi-Sunyer F. X., Thornton J., Smith G. P. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr. 1981 Feb;34(2):154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- Le Sauter J., Geary N. Redundant vagal mediation of the synergistic satiety effect of pancreatic glucagon and cholecystokinin in sham feeding rats. J Auton Nerv Syst. 1990 Apr;30(1):13–22. doi: 10.1016/0165-1838(90)90159-g. [DOI] [PubMed] [Google Scholar]
- Lieverse R. J., Jansen J. B., Lamers C. B. Cholecystokinin and satiation. Neth J Med. 1993 Apr;42(3-4):146–152. [PubMed] [Google Scholar]
- Lieverse R. J., Jansen J. B., van de Zwan A., Samson L., Masclee A. A., Rovati L. C., Lamers C. B. Bombesin reduces food intake in lean man by a cholecystokinin-independent mechanism. J Clin Endocrinol Metab. 1993 Jun;76(6):1495–1498. doi: 10.1210/jcem.76.6.8501156. [DOI] [PubMed] [Google Scholar]
- Lindén A. Role of cholecystokinin in feeding and lactation. Acta Physiol Scand Suppl. 1989;585:i-vii, 1-49. [PubMed] [Google Scholar]
- Makovec F., Chistè R., Bani M., Pacini M. A., Setnikar I., Rovati L. A. New glutaramic acid derivatives with potent competitive and specific cholecystokinin-antagonistic activity. Arzneimittelforschung. 1985;35(7):1048–1051. [PubMed] [Google Scholar]
- McCoy J. G., Stump B., Avery D. D. Intake of individual macronutrients following IP injections of BBS and CCK in rats. Peptides. 1990 Mar-Apr;11(2):221–225. doi: 10.1016/0196-9781(90)90074-f. [DOI] [PubMed] [Google Scholar]
- McHugh P. R., Moran T. H. The stomach, cholecystokinin, and satiety. Fed Proc. 1986 Apr;45(5):1384–1390. [PubMed] [Google Scholar]
- McLaughlin C. L., Baile C. A., Buonomo F. C. Effect of CCK antibodies on food intake and weight gain in Zucker rats. Physiol Behav. 1985 Feb;34(2):277–282. doi: 10.1016/0031-9384(85)90116-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. L., Baile C. A. Decreased sensitivity of Zucker obese rats to the putative satiety agent cholecystokinin. Physiol Behav. 1980 Oct;25(4):543–548. doi: 10.1016/0031-9384(80)90119-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. L., Baile C. A. Feeding response of weanling Zucker obese rats to cholecystokinin and bombesin. Physiol Behav. 1980 Sep;25(3):341–346. doi: 10.1016/0031-9384(80)90270-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. L., Peikin S. R., Baile C. A. Food intake response to modulation of secretion of cholecystokinin in Zucker rats. Am J Physiol. 1983 May;244(5):R676–R685. doi: 10.1152/ajpregu.1983.244.5.R676. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. L., Peikin S. R., Baile C. A. Trypsin inhibitor effects on food intake and weight gain in Zucker rats. Physiol Behav. 1983 Oct;31(4):487–491. doi: 10.1016/0031-9384(83)90071-9. [DOI] [PubMed] [Google Scholar]
- Moran T. H., McHugh P. R. Gastric and nongastric mechanisms for satiety action of cholecystokinin. Am J Physiol. 1988 Apr;254(4 Pt 2):R628–R632. doi: 10.1152/ajpregu.1988.254.4.R628. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer X., Kissileff H. R., Thornton J., Smith G. P. C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol Behav. 1982 Oct;29(4):627–630. doi: 10.1016/0031-9384(82)90230-x. [DOI] [PubMed] [Google Scholar]
- Reidelberger R. D., O'Rourke M. F. Potent cholecystokinin antagonist L 364718 stimulates food intake in rats. Am J Physiol. 1989 Dec;257(6 Pt 2):R1512–R1518. doi: 10.1152/ajpregu.1989.257.6.R1512. [DOI] [PubMed] [Google Scholar]
- Silver A. J., Flood J. F., Song A. M., Morley J. E. Evidence for a physiological role for CCK in the regulation of food intake in mice. Am J Physiol. 1989 Mar;256(3 Pt 2):R646–R652. doi: 10.1152/ajpregu.1989.256.3.R646. [DOI] [PubMed] [Google Scholar]
- Silver A. J., Morley J. E. Role of CCK in regulation of food intake. Prog Neurobiol. 1991;36(1):23–34. doi: 10.1016/0301-0082(91)90035-y. [DOI] [PubMed] [Google Scholar]
- Smith G. P. The therapeutic potential of cholecystokinin. Int J Obes. 1984;8 (Suppl 1):35–38. [PubMed] [Google Scholar]
- Smith G. P., Tyrka A., Gibbs J. Type-A CCK receptors mediate the inhibition of food intake and activity by CCK-8 in 9- to 12-day-old rat pups. Pharmacol Biochem Behav. 1991 Jan;38(1):207–210. doi: 10.1016/0091-3057(91)90612-6. [DOI] [PubMed] [Google Scholar]
- Stacher G. Effects of cholecystokinin and caerulein on human eating behavior and pain sensation: a review. Psychoneuroendocrinology. 1986;11(1):39–48. doi: 10.1016/0306-4530(86)90030-2. [DOI] [PubMed] [Google Scholar]
- Weller A., Smith G. P., Gibbs J. Endogenous cholecystokinin reduces feeding in young rats. Science. 1990 Mar 30;247(4950):1589–1591. doi: 10.1126/science.2321020. [DOI] [PubMed] [Google Scholar]
- West D. B., Fey D., Woods S. C. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984 May;246(5 Pt 2):R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O. M., Gertz B., Weingartner H., Beccaria L., Thompson K., Liddle R. A. Hunger in humans induced by MK-329, a specific peripheral-type cholecystokinin receptor antagonist. Biol Psychiatry. 1990 Jul 15;28(2):169–173. doi: 10.1016/0006-3223(90)90635-f. [DOI] [PubMed] [Google Scholar]


