Abstract
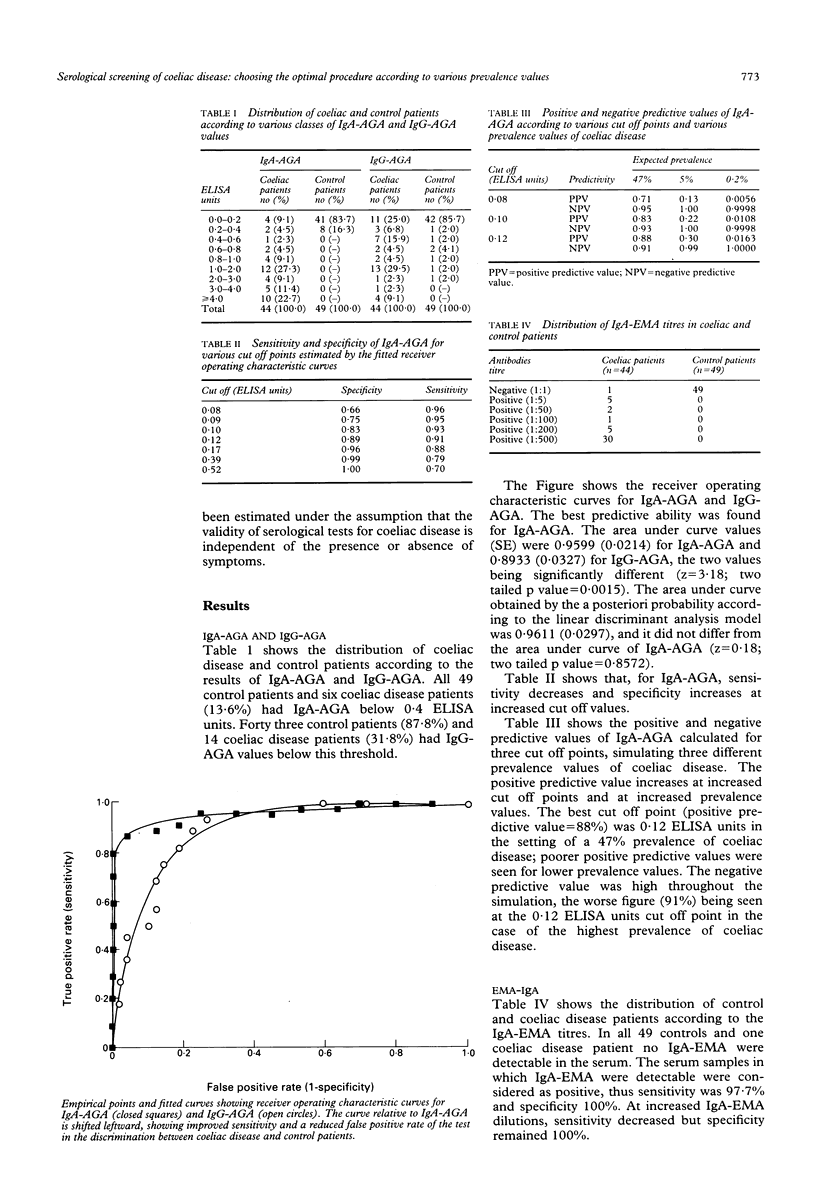
The aim of this study was to select the best approach for screening coeliac disease patients among populations with different grades of disease prevalence. The diagnostic performance was assessed of class A and G antigliadin antibodies and class A antiendomysium antibodies in 93 consecutive outpatients with suspected malabsorption, 44 of whom (47%) had coeliac disease according to duodenal histological tests. Class G antigliadin antibodies provided the worst diagnostic values, whereas a high diagnostic validity was found for the other two tests. The positive predictive value corrected for the disease prevalence expected in coeliac disease relatives (5%) and the general population (0.2%) fell to 30% and < 2% respectively for class A antigliadin antibodies, whereas it remained 100% for antiendomysium antibodies in both situations, providing an optimal value for their use as a screening test and as a valid alternative to duodenal biopsy when this is not feasible. The high cost of anti-endomysium antibodies and the invasive nature of duodenal biopsy prevent them being used widely as screening procedures. A cost effective two step approach was simulated measuring class A antigliadin antibodies in all subjects of the target population (first step), and performing a confirmation test (antiendomysium antibodies or duodenal biopsy) only in subjects positive for antigliadin antibodies. The results show that such a procedure should be recommended only for subjects with an expected low disease prevalence--that is, 5% for coeliac disease relatives and 0.2% for the general population--as the positive predictive value was always 100% with an acceptable false negative rate (6% and 11% respectively), irrespective of which of the two confirmation tests was used. This approach avoids the use of the confirmation test in 63% and 89% of subjects respectively for the two levels of prevalence, resulting in a considerable reduction of the cost. Patients seen for suspected malabsorption with an expected high prevalence of coeliac disease should not have such a serological screening procedure. In conclusion, antigliadin antibodies are useful to screen for asymptomatic coeliac disease in non-hospital communities if antiendomysium anti-bodies are used as a confirmation test: the latter is reasonable valid alternative to duodenal biopsy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cacciari E., Salardi S., Volta U., Biasco G., Lazzari R., Corazza G. R., Feliciani M., Cicognani A., Partesotti S., Azzaroni D. Can antigliadin antibody detect symptomless coeliac disease in children with short stature? Lancet. 1985 Jun 29;1(8444):1469–1471. doi: 10.1016/s0140-6736(85)92251-2. [DOI] [PubMed] [Google Scholar]
- Chorzelski T. P., Beutner E. H., Sulej J., Tchorzewska H., Jablonska S., Kumar V., Kapuscinska A. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984 Oct;111(4):395–402. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- Corazza G. R., Frisoni M., Treggiari E. A., Valentini R. A., Filipponi C., Volta U., Gasbarrini G. Subclinical celiac sprue. Increasing occurrence and clues to its diagnosis. J Clin Gastroenterol. 1993 Jan;16(1):16–21. [PubMed] [Google Scholar]
- Corazza G., Valentini R. A., Frisoni M., Volta U., Corrao G., Bianchi F. B., Gasbarrini G. Gliadin immune reactivity is associated with overt and latent enteropathy in relatives of celiac patients. Gastroenterology. 1992 Nov;103(5):1517–1522. doi: 10.1016/0016-5085(92)91172-z. [DOI] [PubMed] [Google Scholar]
- Ferreira M., Davies S. L., Butler M., Scott D., Clark M., Kumar P. Endomysial antibody: is it the best screening test for coeliac disease? Gut. 1992 Dec;33(12):1633–1637. doi: 10.1136/gut.33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasieri A., Sinico R. A., Maldifassi P., Bernasconi P., Vegni M., D'Amico G. IgA-antigliadin antibodies in IgA mesangial nephropathy (Berger's disease). Br Med J (Clin Res Ed) 1987 Jul 11;295(6590):78–80. doi: 10.1136/bmj.295.6590.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley J. A., McNeil B. J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983 Sep;148(3):839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- Hanley J. A., McNeil B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- Hanley J. A. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging. 1989;29(3):307–335. [PubMed] [Google Scholar]
- Hed J., Lieden G., Ottosson E., Ström M., Walan A., Groth O., Sjögren F., Franzen L. IgA anti-gliadin antibodies and jejunal mucosal lesions in healthy blood donors. Lancet. 1986 Jul 26;2(8500):215–215. doi: 10.1016/s0140-6736(86)92508-0. [DOI] [PubMed] [Google Scholar]
- Hill P. G., Thompson S. P., Holmes G. K. IgA anti-gliadin antibodies in adult celiac disease. Clin Chem. 1991 May;37(5):647–650. [PubMed] [Google Scholar]
- Holmes G. K., Prior P., Lane M. R., Pope D., Allan R. N. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989 Mar;30(3):333–338. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A., Lebenthal E. The controversy of the use of anti-gluten antibody (AGA) as a diagnostic tool in celiac disease. J Pediatr Gastroenterol Nutr. 1991 May;12(4):407–409. doi: 10.1097/00005176-199105000-00001. [DOI] [PubMed] [Google Scholar]
- Logan R. F., Rifkind E. A., Busuttil A., Gilmour H. M., Ferguson A. Prevalence and "incidence" of celiac disease in Edinburgh and the Lothian region of Scotland. Gastroenterology. 1986 Feb;90(2):334–342. doi: 10.1016/0016-5085(86)90929-7. [DOI] [PubMed] [Google Scholar]
- Logan R. F., Rifkind E. A., Turner I. D., Ferguson A. Mortality in celiac disease. Gastroenterology. 1989 Aug;97(2):265–271. doi: 10.1016/0016-5085(89)90060-7. [DOI] [PubMed] [Google Scholar]
- Mazzetti di Pietralata M., Giorgetti G. M., Gregori M., De Simone M., Leonardi C., Barletta P. A., Ricciardi M. M., Sandri G. Subclinical coeliac disease. Ital J Gastroenterol. 1992 Jul-Aug;24(6):352–354. [PubMed] [Google Scholar]
- Mylotte M., Egan-Mitchell B., McCarthy C. F., McNicholl B. Incidence of coeliac disease in the West of Ireland. Br Med J. 1973 Mar 24;1(5855):703–705. doi: 10.1136/bmj.1.5855.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrelly C., Marten D., Melcher D., McDougall B., Price R., Goldstein A. J., Sherwood R., Fernandes L. Association between villous atrophy in rheumatoid arthritis and a rheumatoid factor and gliadin-specific IgG. Lancet. 1988 Oct 8;2(8615):819–822. doi: 10.1016/s0140-6736(88)92784-5. [DOI] [PubMed] [Google Scholar]
- RUBIN C. E., BRANDBORG L. L., PHELPS P. C., TAYLOR H. C., Jr Studies of celiac disease. I. The apparent identical and specific nature of the duodenal and proximal jejunal lesion in celiac disease and idiopathic sprue. Gastroenterology. 1960 Jan;38:28–49. [PubMed] [Google Scholar]
- Ritchie K., Fuhrer R. A comparative study of the performance of screening tests for senile dementia using receiver operating characteristics analysis. J Clin Epidemiol. 1992 Jun;45(6):627–637. doi: 10.1016/0895-4356(92)90135-a. [DOI] [PubMed] [Google Scholar]
- Savilahti E., Simell O., Koskimies S., Rilva A., Akerblom H. K. Celiac disease in insulin-dependent diabetes mellitus. J Pediatr. 1986 May;108(5 Pt 1):690–693. doi: 10.1016/s0022-3476(86)81042-3. [DOI] [PubMed] [Google Scholar]
- Stenhammar L., Brandt A., Wågermark J. A family study of coeliac disease. Acta Paediatr Scand. 1982 Jul;71(4):625–628. doi: 10.1111/j.1651-2227.1982.tb09486.x. [DOI] [PubMed] [Google Scholar]
- Swets J. A. Form of empirical ROCs in discrimination and diagnostic tasks: implications for theory and measurement of performance. Psychol Bull. 1986 Mar;99(2):181–198. [PubMed] [Google Scholar]
- Swets J. A., Pickett R. M., Whitehead S. F., Getty D. J., Schnur J. A., Swets J. B., Freeman B. A. Assessment of diagnostic technologies. Science. 1979 Aug 24;205(4408):753–759. doi: 10.1126/science.462188. [DOI] [PubMed] [Google Scholar]
- Swinson C. M., Levi A. J. Is coeliac disease underdiagnosed? Br Med J. 1980 Nov 8;281(6250):1258–1260. doi: 10.1136/bmj.281.6250.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncone R., Ferguson A. Anti-gliadin antibodies. J Pediatr Gastroenterol Nutr. 1991 Feb;12(2):150–158. doi: 10.1097/00005176-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Volta U., Lenzi M., Lazzari R., Cassani F., Collina A., Bianchi F. B., Pisi E. Antibodies to gliadin detected by immunofluorescence and a micro-ELISA method: markers of active childhood and adult coeliac disease. Gut. 1985 Jul;26(7):667–671. doi: 10.1136/gut.26.7.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volta U., Molinaro N., Fusconi M., Cassani F., Bianchi F. B. IgA antiendomysial antibody test. A step forward in celiac disease screening. Dig Dis Sci. 1991 Jun;36(6):752–756. doi: 10.1007/BF01311232. [DOI] [PubMed] [Google Scholar]


