Abstract
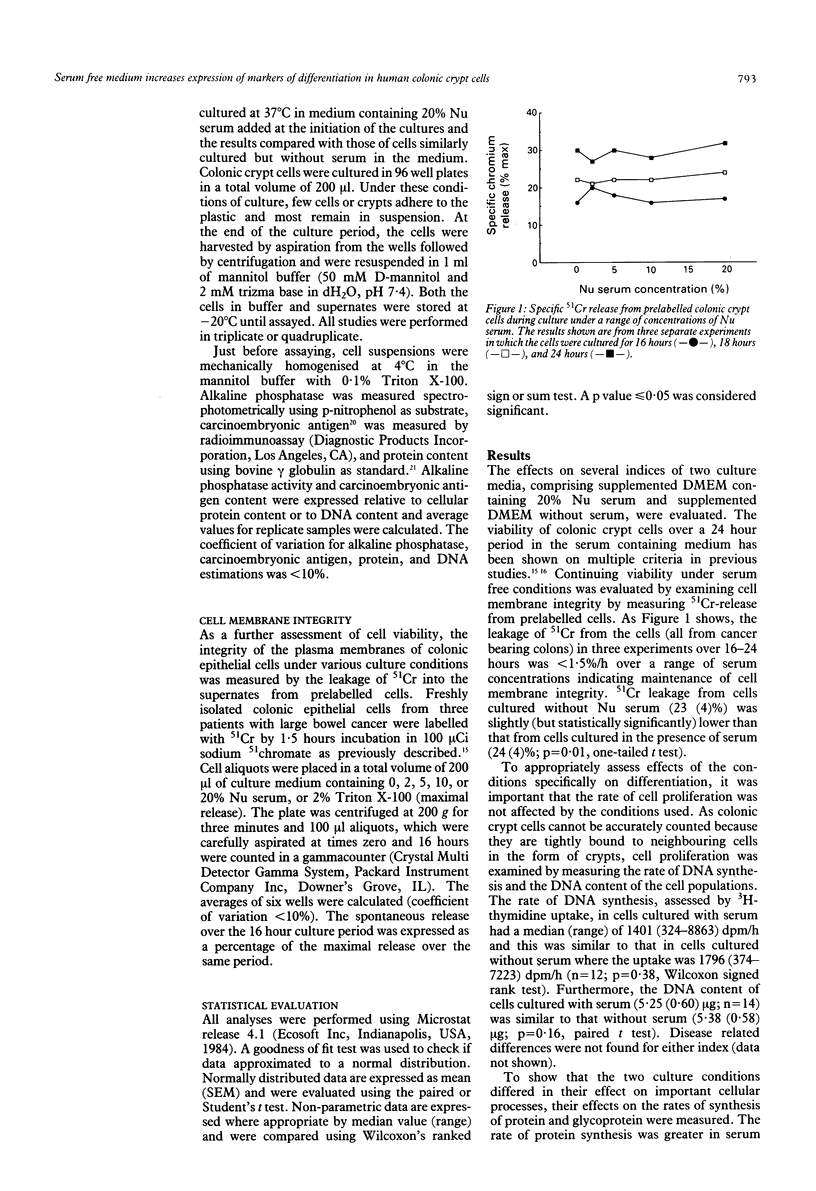
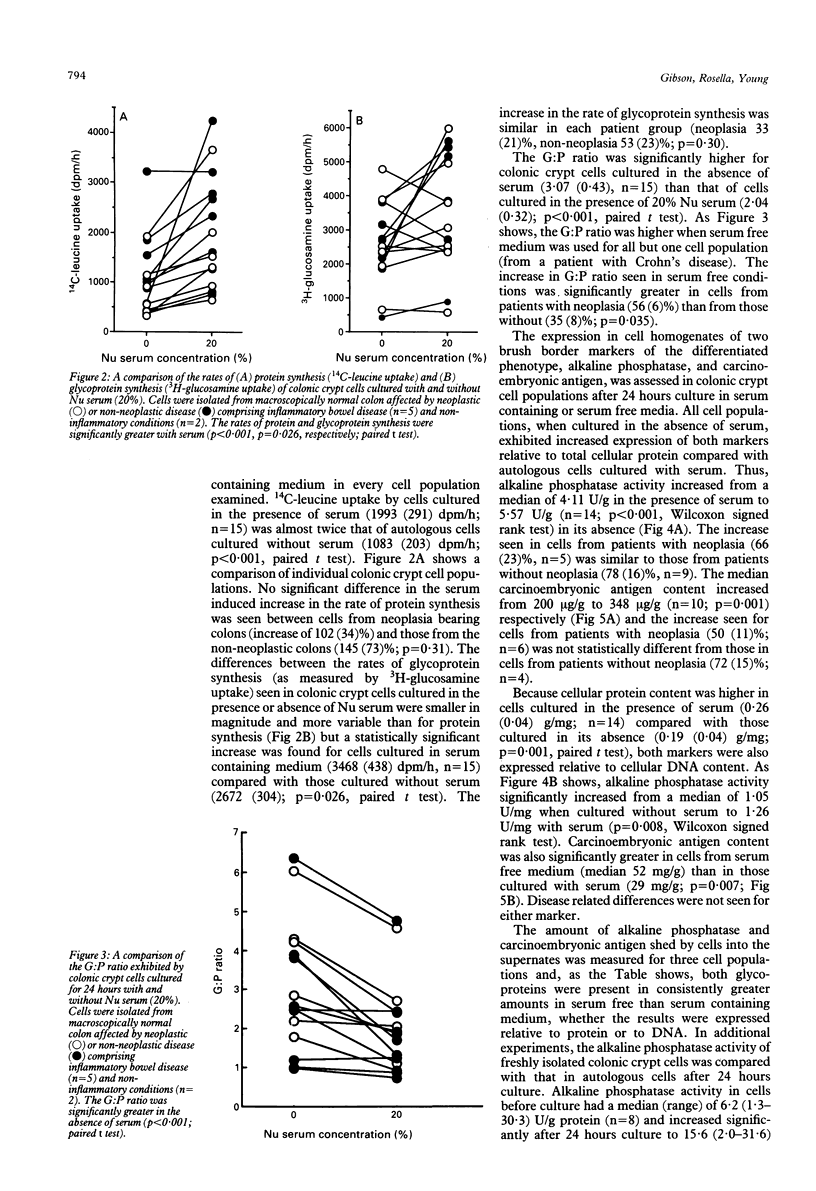
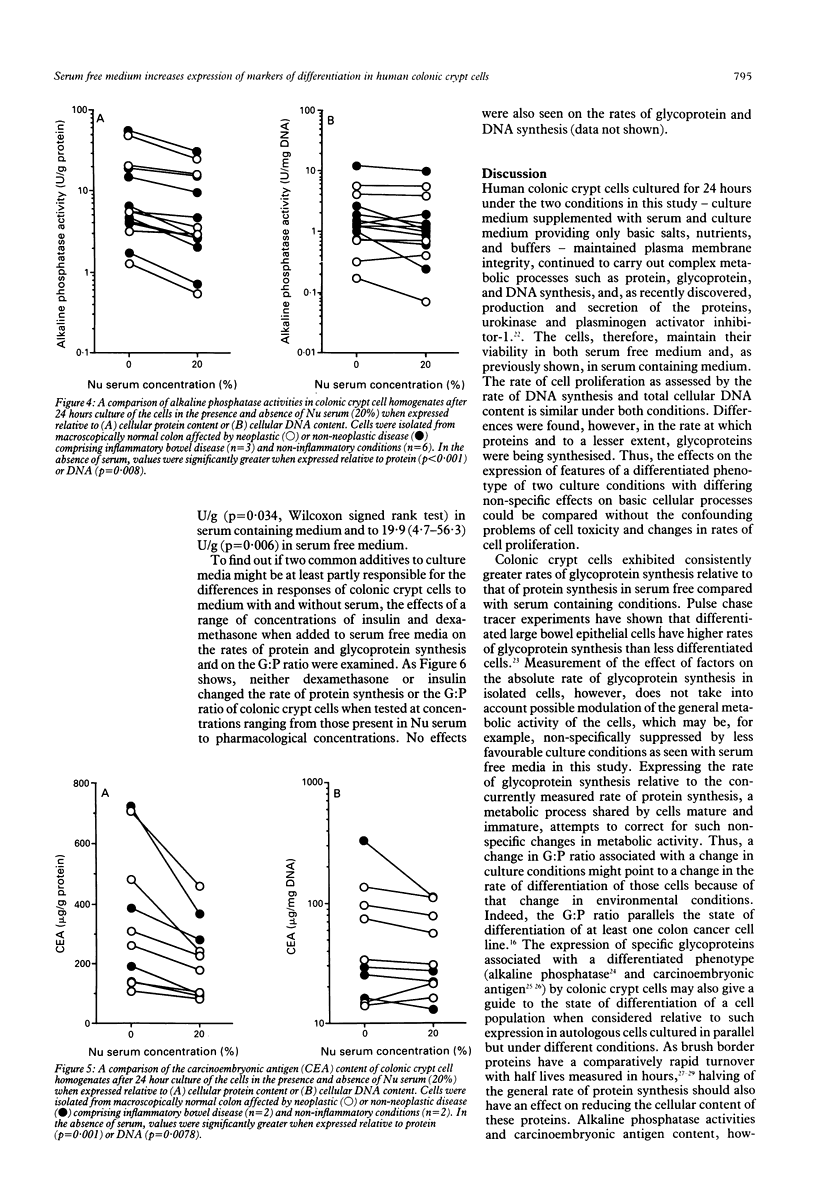
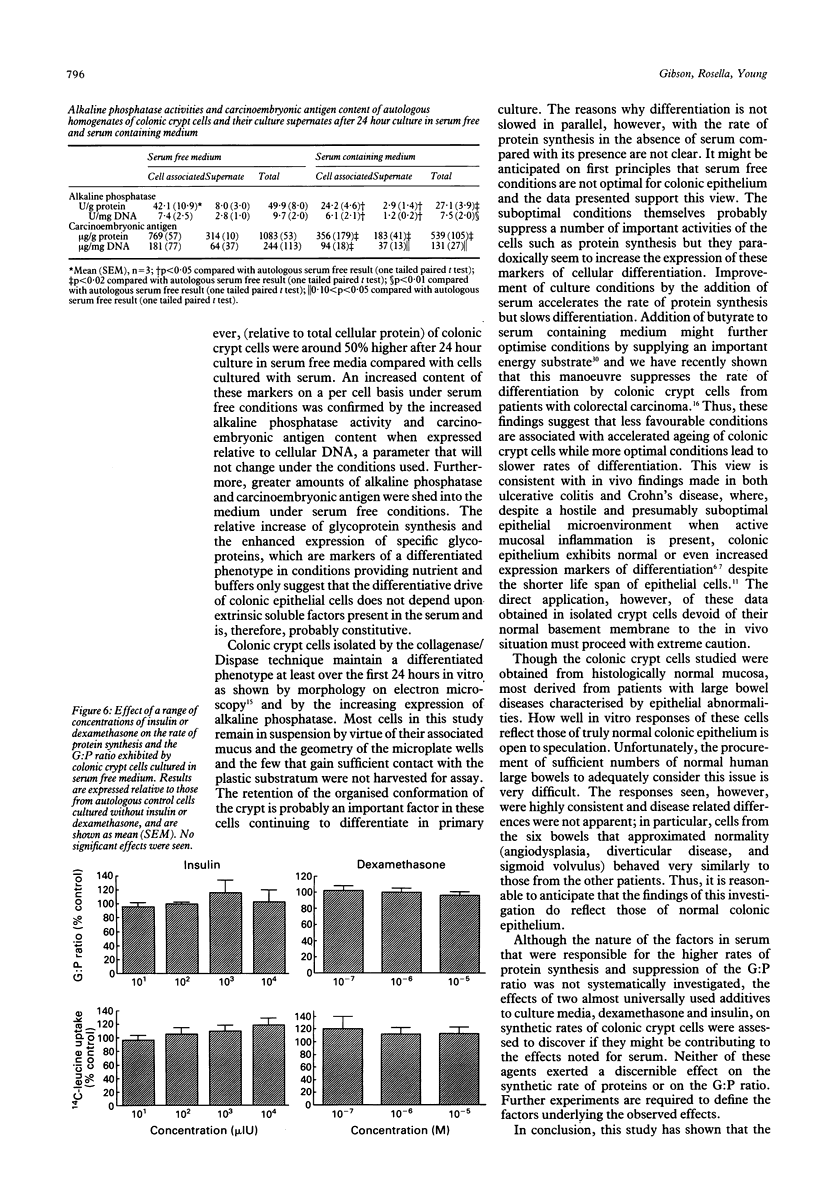
In colitis, colonic epithelial cells have a shortened life span but show normal or increased expression of phenotypic markers of differentiation. This study examined the effect of differing culture conditions on the expression of such markers in colonic crypt cells. Crypt cells were enzymatically isolated from macroscopically normal large bowel mucosa resected because of neoplasia, inflammatory bowel disease or non-neoplastic non-inflammatory conditions. Cells cultured in the presence of serum exhibited a doubling of the rate of protein synthesis (measured by 14C-leucine uptake; p < 0.001) compared with autologous cells cultured in the absence of serum without evidence of loss of cell viability (assessed by 51Cr release from prelabelled cells) or of change in the rate of cell proliferation (assessed by total DNA content and 3H-thymidine uptake). Irrespective of the underlying colonic disease, crypt cells cultured in the absence of serum exhibited increased expression of phenotypic markers of differentiation compared with those cultured with serum: the rate of glycoprotein synthesis relative to that of protein synthesis increased by a mean of 59% and the cellular expression of brush border glycoproteins, alkaline phosphatase, and carcinoembryonic antigen significantly increased. The effects seen could not be mimicked by addition of dexamethasone or insulin to serum free medium. Thus, under less optimal (serum free) culture conditions, colonic crypt cells express phenotypic markers of differentiation at an accelerated rate suggesting that unfavourable microenvironmental conditions themselves are probably in part responsible for the normal or increased expression of such markers in colitis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Kinoshita K., Nakane P. K., Brown W. R. Differential expression carcinoembryonic antigen and secretory component during colonic epithelial cell differentiation and in colonic carcinomas. Gastroenterology. 1987 Dec;93(6):1330–1338. doi: 10.1016/0016-5085(87)90263-0. [DOI] [PubMed] [Google Scholar]
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H. The relation of size to the relative rates of degradation of intestinal brush border proteins. J Clin Invest. 1972 Oct;51(10):2621–2630. doi: 10.1172/JCI107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste E. J., Rizack M. A. In vitro cyclic AMP-mediated lipolytic activity of endorphins, enkephalins and naloxone. Life Sci. 1980 Jul 14;27(2):135–141. doi: 10.1016/0024-3205(80)90455-5. [DOI] [PubMed] [Google Scholar]
- Bell L., Williams L. Histochemical demonstration of alkaline phosphatase in human large intestine, normal and diseased. Histochemistry. 1979 Feb 26;60(1):85–89. doi: 10.1007/BF00495731. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cooper H. S., Steplewski Z. Immunohistologic study of ulcerative colitis with monoclonal antibodies against tumor-associated and/or differentiation antigens. Gastroenterology. 1988 Sep;95(3):686–693. doi: 10.1016/s0016-5085(88)80015-5. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Trier J. S. Epithelial cell renewal in cultured rectal biopsies in ulcerative colitis. Gastroenterology. 1973 Mar;64(3):383–390. [PubMed] [Google Scholar]
- Gibson P. R., Folino M., Rosella O., Finch C. F., Moeller I., Alexeyeff M., Lindley J., Young G. P. Neoplasia and hyperplasia of large bowel: focal lesions in an abnormal epithelium. Gastroenterology. 1992 Nov;103(5):1452–1459. doi: 10.1016/0016-5085(92)91164-y. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Moeller I., Kagelari O., Folino M., Young G. P. Contrasting effects of butyrate on the expression of phenotypic markers of differentiation in neoplastic and non-neoplastic colonic epithelial cells in vitro. J Gastroenterol Hepatol. 1992 Mar-Apr;7(2):165–172. doi: 10.1111/j.1440-1746.1992.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Pavli P. Pathogenic factors in inflammatory bowel disease. I. Ulcerative colitis. Dig Dis. 1992;10(1):17–28. doi: 10.1159/000171340. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., van de Pol E., Maxwell L. E., Gabriel A., Doe W. F. Isolation of colonic crypts that maintain structural and metabolic viability in vitro. Gastroenterology. 1989 Feb;96(2 Pt 1):283–291. doi: 10.1016/0016-5085(89)91549-7. [DOI] [PubMed] [Google Scholar]
- Greenstein A. J., Panvelliwalla D. K., Katz L. B., Heimann T. M., Donnelly J., Pertsimlidis D., Geller S., Smith H., Aufses A. H., Jr Tissue carcinoembryonic antigen, dysplasia, and disease duration in colonic inflammatory bowel disease. Am J Gastroenterol. 1982 Apr;77(4):212–215. [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Freiburghaus A., Grenier J. F., Hadorn B. Biosynthesis of brush border glycoproteins by human small intestinal mucosa in organ culture. Biochim Biophys Acta. 1977 Jun 16;467(3):327–339. doi: 10.1016/0005-2736(77)90310-8. [DOI] [PubMed] [Google Scholar]
- Haviland A. E., Borowitz M. J., Lan M. S., Kaufman B., Khorrami A., Phelps P. C., Metzgar R. S. Aberrant expression of monoclonal antibody-defined colonic mucosal antigens in inflammatory bowel disease. Gastroenterology. 1988 Nov;95(5):1302–1311. doi: 10.1016/0016-5085(88)90365-4. [DOI] [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. P., Alpers D. H., Gerber J. E., Isselbacher K. J. The turnover of disaccharidases and brush border proteins in rat intestine. Biochim Biophys Acta. 1971 Feb 23;230(2):194–203. doi: 10.1016/0304-4165(71)90204-2. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Grand R. J., Trier J. S. Glycoprotein synthesis, transport, and secretion by epithelial cells of human rectal mucosa: normal and cystic fibrosis. Lab Invest. 1977 May;36(5):535–546. [PubMed] [Google Scholar]
- Primus F. J., Clark C. A., Goldenburg D. M. Immunoperoxidase localization of carcinoembryonic antigen in normal human intestinal mucosa. J Natl Cancer Inst. 1981 Nov;67(5):1031–1039. [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. The starved colon--diminished mucosal nutrition, diminished absorption, and colitis. Dis Colon Rectum. 1990 Oct;33(10):858–862. doi: 10.1007/BF02051922. [DOI] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead R. H., Brown A., Bhathal P. S. A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev Biol. 1987 Jun;23(6):436–442. doi: 10.1007/BF02623860. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Young G. P., Bhathal P. S. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215). Gut. 1986 Dec;27(12):1457–1463. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. C., D'Emilia J. C., Salem R. R., DeCoste D., Sears H. F., Gottlieb L. S., Steele G. D., Jr Detection of the tumor-associated glycoprotein antigen (TAG-72) in premalignant lesions of the colon. J Natl Cancer Inst. 1989 Dec 20;81(24):1913–1917. doi: 10.1093/jnci/81.24.1913. [DOI] [PubMed] [Google Scholar]
- Young G. P., Macrae F. A., Gibson P. R., Alexeyeff M., Whitehead R. H. Brush border hydrolases in normal and neoplastic colonic epithelium. J Gastroenterol Hepatol. 1992 Jul-Aug;7(4):347–354. doi: 10.1111/j.1440-1746.1992.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Young G. P., Rose I. S., Cropper S., Seetharam S., Alpers D. H. Hepatic clearance of rat plasma intestinal alkaline phosphatase. Am J Physiol. 1984 Oct;247(4 Pt 1):G419–G426. doi: 10.1152/ajpgi.1984.247.4.G419. [DOI] [PubMed] [Google Scholar]


