Abstract
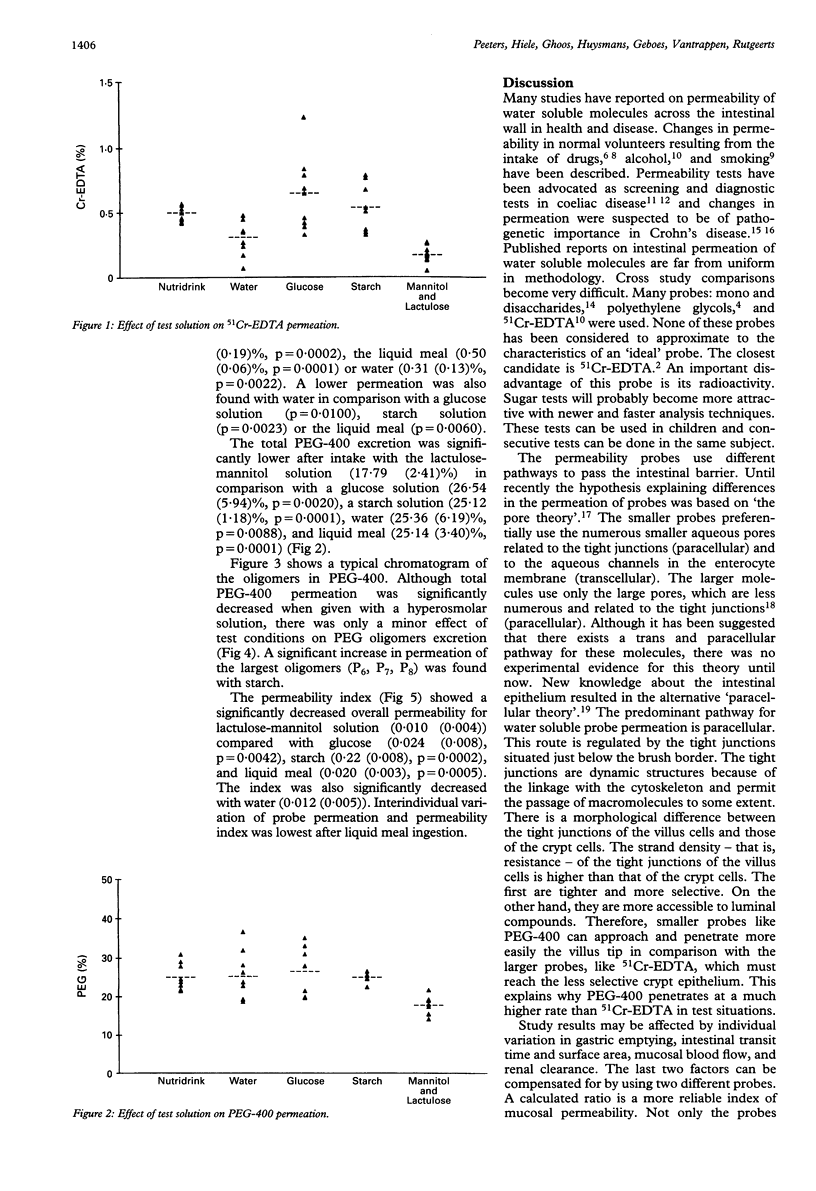
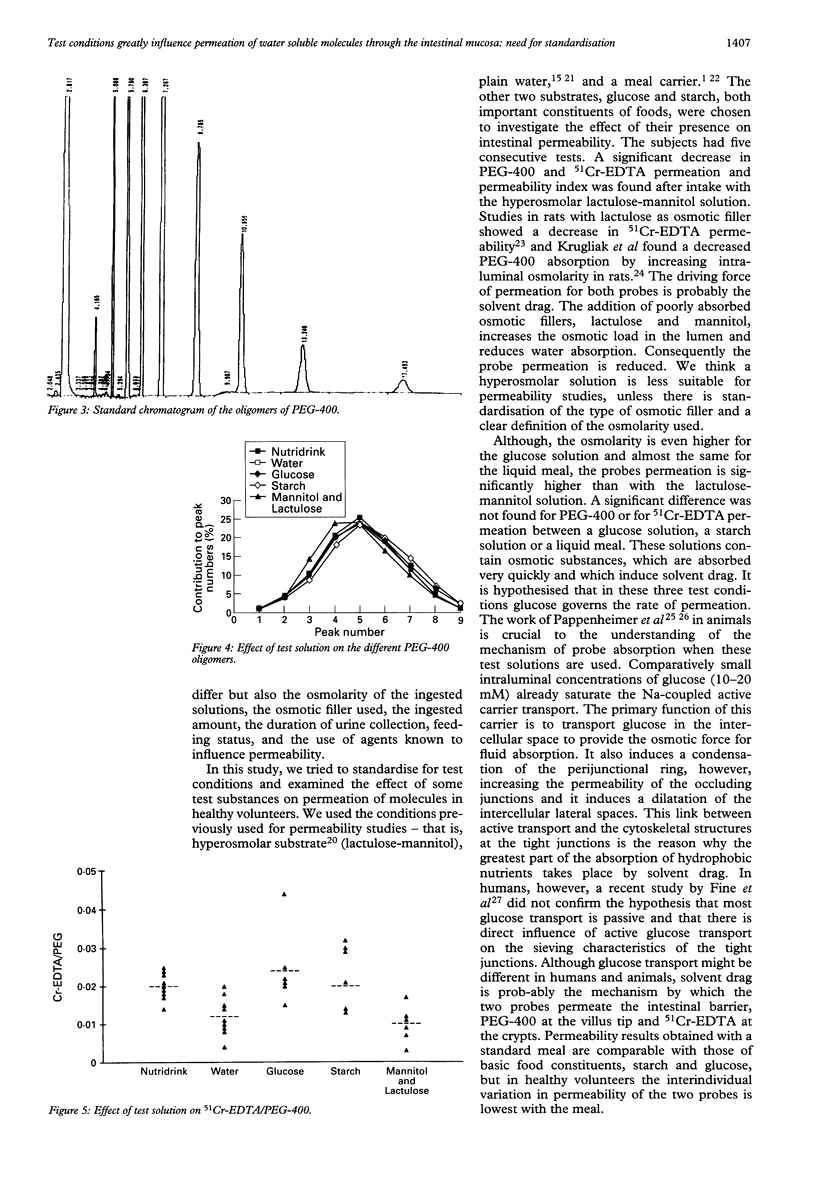
Permeability tests are widely used to investigate the pathogenesis of various gastrointestinal diseases including coeliac disease, infectious diarrhoea, and inflammatory bowel disease. In Crohn's disease they are used as activity parameters by some investigators. Lack of standardisation, however, makes it very difficult to compare data reported in different studies. The aim of this study was to gather permeation data in well controlled test conditions to standardise the methods. Nine healthy volunteers each received five consecutive permeability tests by mouth using polyethylene glycol-400 (PEG-400) and 51Cr-EDTA as probe molecules. The probes were dissolved in water, a glucose solution, a starch solution, a hyperosmolar lactulose-mannitol solution, and a liquid meal. A significantly decreased permeation for both probes was found when given with the hyperosmolar solution. The 51Cr-EDTA permeation was also decreased with water. The permeability index, 51Cr-EDTA/PEG-400, corrected for influencing factors, confirmed that the lactulose-mannitol solution and plain water yield lower values of macro-molecule permeation than starch, glucose or liquid meal. Hyperosmolarity was clearly accompanied by a decrease in permeability probably caused by reversed solvent drag. Interindividual variability of probe permeation and permeability index is very low with a standard liquid meal. It is proposed that for permeability studies a standard liquid meal is always used.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aabakken L. Cr-ethylenediaminetetraacetic acid absorption test. Methodologic aspects. Scand J Gastroenterol. 1989 Apr;24(3):351–358. doi: 10.3109/00365528909093059. [DOI] [PubMed] [Google Scholar]
- Ainsworth M., Eriksen J., Rasmussen J. W., Schaffalitzky de Muckadell O. B. Intestinal permeability of 51Cr-labelled ethylenediaminetetraacetic acid in patients with Crohn's disease and their healthy relatives. Scand J Gastroenterol. 1989 Oct;24(8):993–998. doi: 10.3109/00365528909089246. [DOI] [PubMed] [Google Scholar]
- Andre C., Descos L., Minaire Y. Comparison between lactulose-mannitol test and 51Cr-labelled ethylene diamine tetra-acetate test in inflammatory bowel diseases. Hepatogastroenterology. 1990 Dec;37 (Suppl 2):113–117. [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Levi A. J., Peters T. J. Intestinal permeability to 51Cr-EDTA in rats with experimentally induced enteropathy. Gut. 1985 Jun;26(6):579–585. doi: 10.1136/gut.26.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). I. Chemical analysis and biological properties of PEG 400. Gastroenterology. 1977 Aug;73(2):241–246. [PubMed] [Google Scholar]
- Cooper B. T. Small intestinal permeability in clinical practice. J Clin Gastroenterol. 1984 Dec;6(6):499–501. [PubMed] [Google Scholar]
- Elia M., Behrens R., Northrop C., Wraight P., Neale G. Evaluation of mannitol, lactulose and 51Cr-labelled ethylenediaminetetra-acetate as markers of intestinal permeability in man. Clin Sci (Lond) 1987 Aug;73(2):197–204. doi: 10.1042/cs0730197. [DOI] [PubMed] [Google Scholar]
- Fine K. D., Santa Ana C. A., Porter J. L., Fordtran J. S. Effect of D-glucose on intestinal permeability and its passive absorption in human small intestine in vivo. Gastroenterology. 1993 Oct;105(4):1117–1125. doi: 10.1016/0016-5085(93)90957-e. [DOI] [PubMed] [Google Scholar]
- Fleming S. C., Kynaston J. A., Laker M. F., Pearson A. D., Kapembwa M. S., Griffin G. E. Analysis of multiple sugar probes in urine and plasma by high-performance anion-exchange chromatography with pulsed electrochemical detection. Application in the assessment of intestinal permeability in human immunodeficiency virus infection. J Chromatogr. 1993 Jun 25;640(1-2):293–297. doi: 10.1016/0021-9673(93)80193-c. [DOI] [PubMed] [Google Scholar]
- Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992 Sep;27(9):721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- Hollander D., Vadheim C. M., Brettholz E., Petersen G. M., Delahunty T., Rotter J. I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Howden C. W., Robertson C., Duncan A., Morris A. J., Russell R. I. Comparison of different measurements of intestinal permeability in inflammatory bowel disease. Am J Gastroenterol. 1991 Oct;86(10):1445–1449. [PubMed] [Google Scholar]
- Jenkins A. P., Trew D. R., Crump B. J., Nukajam W. S., Foley J. A., Menzies I. S., Creamer B. Do non-steroidal anti-inflammatory drugs increase colonic permeability? Gut. 1991 Jan;32(1):66–69. doi: 10.1136/gut.32.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. T., Goodacre R. L., Rooney P. J., Bienenstock J., Sivakumaran T., Walker W. H. Studies of intestinal permeability in inflammatory diseases using polyethylene glycol 400. Clin Biochem. 1986 Oct;19(5):298–302. doi: 10.1016/s0009-9120(86)80045-5. [DOI] [PubMed] [Google Scholar]
- Jenkins R. T., Jones D. B., Goodacre R. L., Collins S. M., Coates G., Hunt R. H., Bienenstock J. Reversibility of increased intestinal permeability to 51Cr-EDTA in patients with gastrointestinal inflammatory diseases. Am J Gastroenterol. 1987 Nov;82(11):1159–1164. [PubMed] [Google Scholar]
- Juby L. D., Rothwell J., Axon A. T. Lactulose/mannitol test: an ideal screen for celiac disease. Gastroenterology. 1989 Jan;96(1):79–85. doi: 10.1016/0016-5085(89)90767-1. [DOI] [PubMed] [Google Scholar]
- Krugliak P., Hollander D., Le K., Ma T., Dadufalza V. D., Katz K. D. Regulation of polyethylene glycol 400 intestinal permeability by endogenous and exogenous prostanoids. Influence of non-steroidal anti-inflammatory drugs. Gut. 1990 Apr;31(4):417–421. doi: 10.1136/gut.31.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugliak P., Hollander D., Ma T. Y., Tran D., Dadufalza V. D., Katz K. D., Le K. Mechanisms of polyethylene glycol 400 permeability of perfused rat intestine. Gastroenterology. 1989 Nov;97(5):1164–1170. doi: 10.1016/0016-5085(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Lifschitz C. H., Mahoney D. H. Low-dose methotrexate-induced changes in intestinal permeability determined by polyethylene glycol polymers. J Pediatr Gastroenterol Nutr. 1989 Oct;9(3):301–306. doi: 10.1097/00005176-198910000-00007. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Pappenheimer J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Martines D., Morris A. I., Gilmore I. T., Williams A., Stockdale H., Critchley M., Smith G. A., Billington D. Comparison between the cellobiose/mannitol and 51Cr-labelled ethylenediaminetetra-acetate absorption tests in the detection of coeliac disease. Clin Sci (Lond) 1988 Oct;75(4):375–378. doi: 10.1042/cs0750375. [DOI] [PubMed] [Google Scholar]
- Maxton D. G., Bjarnason I., Reynolds A. P., Catt S. D., Peters T. J., Menzies I. S. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 1986 Jul;71(1):71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- May G. R., Sutherland L. R., Meddings J. B. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology. 1993 Jun;104(6):1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R. Paracellular intestinal absorption of glucose, creatinine, and mannitol in normal animals: relation to body size. Am J Physiol. 1990 Aug;259(2 Pt 1):G290–G299. doi: 10.1152/ajpgi.1990.259.2.G290. [DOI] [PubMed] [Google Scholar]
- Philipsen E. K., Batsberg W., Christensen A. B. Gastrointestinal permeability to polyethylene glycol: an evaluation of urinary recovery of an oral load of polyethylene glycol as a parameter of intestinal permeability in man. Eur J Clin Invest. 1988 Apr;18(2):139–145. doi: 10.1111/j.1365-2362.1988.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Pironi L., Miglioli M., Ruggeri E., Levorato M., Dallasta M. A., Corbelli C., Nibali M. G., Barbara L. Relationship between intestinal permeability to [51Cr]EDTA and inflammatory activity in asymptomatic patients with Crohn's disease. Dig Dis Sci. 1990 May;35(5):582–588. doi: 10.1007/BF01540405. [DOI] [PubMed] [Google Scholar]
- Söderholm J., Olaison G., Sjödahl R., Tagesson C. Smoking and intestinal absorption of oral polyethylene glycols in Crohn's disease. Scand J Gastroenterol. 1993 Feb;28(2):163–167. doi: 10.3109/00365529309096064. [DOI] [PubMed] [Google Scholar]
- Teahon K., Smethurst P., Levi A. J., Menzies I. S., Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992 Mar;33(3):320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S., Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond) 1992 May;82(5):471–488. doi: 10.1042/cs0820471. [DOI] [PubMed] [Google Scholar]


