Abstract
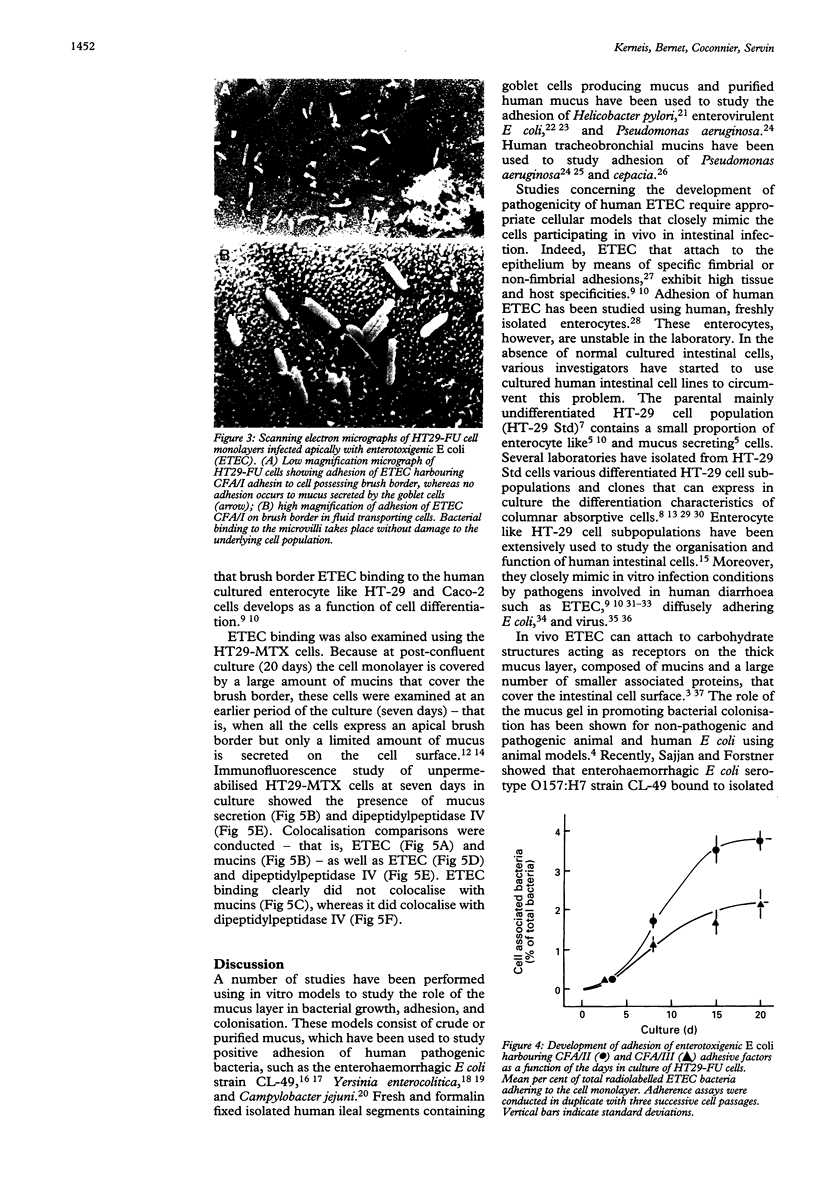
Enterotoxigenic Escherichia coli (ETEC) bearing the fimbrial colonisation factor antigens CFA/I, CFA/II, CFA/III, and the non-fimbrial antigen 2230 were tested for their ability to adhere to two cultured human intestinal HT-29 mucus secreting cell subpopulations. These populations are referred to as HT29-MTX and HT29-FU, which differ in the amount of secreted mucins and in their gastric or colonic mucin immunoreactivity respectively. Adherence of radiolabelled bacteria to cell monolayers infected apically was assessed. All ETEC strains adhered to the mucus secreting HT29-FU subpopulation, which secretes mucins of colonic immunoreactivity. Visualisation of bacteria by scanning electron microscopy showed that ETEC bound to the HT29-FU cells possessing a brush border, but not to the mucus and that ETEC binding developed as a function of cell differentiation. The adhesion of ETEC to cells possessing a brush border and to mucus secreting cells was also analysed by indirect immunofluorescence in HT29-MTX cells, which secrete mucins of gastric immunoreactivity. Fluorescein isothiocyanate labelling using specific anti-CFA/I antibody was used to show ETEC; rhodamine isothiocyanate labelling using a monoclonal antibody (designated M1) against purified human gastric mucus was used to detect secreted mucins, and rhodamine isothiocyanate labelling using a monoclonal antibody (designated 4H3) against human dipeptidylpeptidase IV was used to show cells possessing a brush border. Binding of bacteria colocalised with dipeptidylpeptidase IV of enterocytes and not with mucins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augeron C., Laboisse C. L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984 Sep;44(9):3961–3969. [PubMed] [Google Scholar]
- Bara J., Gautier R., Daher N., Zaghouani H., Decaens C. Monoclonal antibodies against oncofetal mucin M1 antigens associated with precancerous colonic mucosae. Cancer Res. 1986 Aug;46(8):3983–3989. [PubMed] [Google Scholar]
- Dahiya R., Lesuffleur T., Kwak K. S., Byrd J. C., Barbat A., Zweibaum A., Kim Y. S. Expression and characterization of mucins associated with the resistance to methotrexate of human colonic adenocarcinoma cell line HT29. Cancer Res. 1992 Sep 1;52(17):4655–4662. [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Aubel D., Chauviere G., Rich C., Bourges M., Servin A., Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990 Apr;58(4):893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Isberg R. R., Portnoy D. A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- Fantini J., Yahi N., Chermann J. C. Human immunodeficiency virus can infect the apical and basolateral surfaces of human colonic epithelial cells. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9297–9301. doi: 10.1073/pnas.88.20.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Hafez M. M., Infante D., Winawer S., Friedman E. Transforming growth factor beta 1 acts as an autocrine-negative growth regulator in colon enterocytic differentiation but not in goblet cell maturation. Cell Growth Differ. 1990 Dec;1(12):617–626. [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry A., Muzeau F., Laboisse C. Cytokine effects in a human colonic goblet cell line. Cellular damage and its partial prevention by 5 aminosalicylic acid. Dig Dis Sci. 1992 Aug;37(8):1170–1178. doi: 10.1007/BF01296556. [DOI] [PubMed] [Google Scholar]
- Kerneis S., Bilge S. S., Fourel V., Chauviere G., Coconnier M. H., Servin A. L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991 Nov;59(11):4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernéis S., Chauvière G., Darfeuille-Michaud A., Aubel D., Coconnier M. H., Joly B., Servin A. L. Expression of receptors for enterotoxigenic Escherichia coli during enterocytic differentiation of human polarized intestinal epithelial cells in culture. Infect Immun. 1992 Jul;60(7):2572–2580. doi: 10.1128/iai.60.7.2572-2580.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., Candy D. C., McNeish A. S. Adhesion of enterotoxigenic Escherichia coli to human small intestinal enterocytes. Infect Immun. 1985 Jun;48(3):824–831. doi: 10.1128/iai.48.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991 Jul-Aug;13(4):721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T., Barbat A., Dussaulx E., Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990 Oct 1;50(19):6334–6343. [PubMed] [Google Scholar]
- Lesuffleur T., Porchet N., Aubert J. P., Swallow D., Gum J. R., Kim Y. S., Real F. X., Zweibaum A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993 Nov;106(Pt 3):771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- Mann E. A., Cohen M. B., Giannella R. A. Comparison of receptors for Escherichia coli heat-stable enterotoxin: novel receptor present in IEC-6 cells. Am J Physiol. 1993 Jan;264(1 Pt 1):G172–G178. doi: 10.1152/ajpgi.1993.264.1.G172. [DOI] [PubMed] [Google Scholar]
- Mantle M., Basaraba L., Peacock S. C., Gall D. G. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect Immun. 1989 Nov;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoret J. J., Font J., Augeron C., Codogno P., Bauvy C., Aubery M., Laboisse C. L. A mucus-secreting human colonic cancer cell line. Purification and partial characterization of the secreted mucins. Biochem J. 1989 Mar 15;258(3):793–799. doi: 10.1042/bj2580793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Burr D. H., Walker R. I. Intestinal mucus gel and secretory antibody are barriers to Campylobacter jejuni adherence to INT 407 cells. Infect Immun. 1987 Jun;55(6):1431–1435. doi: 10.1128/iai.55.6.1431-1435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micots I., Augeron C., Laboisse C. L., Muzeau F., Mégraud F. Mucin exocytosis: a major target for Helicobacter pylori. J Clin Pathol. 1993 Mar;46(3):241–245. doi: 10.1136/jcp.46.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Chambaz A., Golliard M., Link-Amster H., Fryder V., Kolodziejczyk E. Adhesion of colonization factor antigen II-positive enterotoxigenic Escherichia coli strains to human enterocytelike differentiated HT-29 cells: a basis for host-pathogen interactions in the gut. Infect Immun. 1989 Dec;57(12):3727–3734. doi: 10.1128/iai.57.12.3727-3734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orø H. S., Kolstø A. B., Wennerås C., Svennerholm A. M. Identification of asialo GM1 as a binding structure for Escherichia coli colonization factor antigens. FEMS Microbiol Lett. 1990 Nov;60(3):289–292. doi: 10.1016/0378-1097(90)90319-l. [DOI] [PubMed] [Google Scholar]
- Paerregaard A., Espersen F., Jensen O. M., Skurnik M. Interactions between Yersinia enterocolitica and rabbit ileal mucus: growth, adhesion, penetration, and subsequent changes in surface hydrophobicity and ability to adhere to ileal brush border membrane vesicles. Infect Immun. 1991 Jan;59(1):253–260. doi: 10.1128/iai.59.1.253-260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T. E., Huet C., Bilbo P. R., Podolsky D. K., Louvard D., Neutra M. R. Human intestinal goblet cells in monolayer culture: characterization of a mucus-secreting subclone derived from the HT29 colon adenocarcinoma cell line. Gastroenterology. 1988 Jun;94(6):1390–1403. doi: 10.1016/0016-5085(88)90678-6. [DOI] [PubMed] [Google Scholar]
- Reddy M. S. Human tracheobronchial mucin: purification and binding to Pseudomonas aeruginosa. Infect Immun. 1992 Apr;60(4):1530–1535. doi: 10.1128/iai.60.4.1530-1535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi N., Laburthe M., Fleming N., Crowther R., Forstner J. Cholera-induced mucin secretion from rat intestine: lack of effect of cAMP, cycloheximide, VIP, and colchicine. Am J Physiol. 1984 Aug;247(2 Pt 1):G140–G148. doi: 10.1152/ajpgi.1984.247.2.G140. [DOI] [PubMed] [Google Scholar]
- Roumagnac I., Laboisse C. A mucus-secreting human colonic epithelial cell line responsive to cholinergic stimulation. Biol Cell. 1987;61(1-2):65–68. doi: 10.1111/j.1768-322x.1987.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Characteristics of binding of Escherichia coli serotype O157:H7 strain CL-49 to purified intestinal mucin. Infect Immun. 1990 Apr;58(4):860–867. doi: 10.1128/iai.58.4.860-867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Identification of the mucin-binding adhesin of Pseudomonas cepacia isolated from patients with cystic fibrosis. Infect Immun. 1992 Apr;60(4):1434–1440. doi: 10.1128/iai.60.4.1434-1440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Role of the putative "link" glycopeptide of intestinal mucin in binding of piliated Escherichia coli serotype O157:H7 strain CL-49. Infect Immun. 1990 Apr;58(4):868–873. doi: 10.1128/iai.58.4.868-873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U., Reisman J., Doig P., Irvin R. T., Forstner G., Forstner J. Binding of nonmucoid Pseudomonas aeruginosa to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992 Feb;89(2):657–665. doi: 10.1172/JCI115632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti F., Tinari A., Baldassarri L., Donelli G. HT-29 cells: a new substrate for rotavirus growth. Arch Virol. 1991;116(1-4):159–173. doi: 10.1007/BF01319239. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis L. S., Mentis A. F., Makris A. M., Spiliadis C., Blackwell C., Weir D. M. In vitro binding of Helicobacter pylori to human gastric mucin. Infect Immun. 1991 Nov;59(11):4252–4254. doi: 10.1128/iai.59.11.4252-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerås C., Holmgren J., Svennerholm A. M. The binding of colonization factor antigens of enterotoxigenic Escherichia coli to intestinal cell membrane proteins. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):107–112. doi: 10.1016/0378-1097(90)90266-s. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Endo S., Yokota T., Echeverria P. Characteristics of adherence of enteroaggregative Escherichia coli to human and animal mucosa. Infect Immun. 1991 Oct;59(10):3722–3739. doi: 10.1128/iai.59.10.3722-3739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Fujita K., Yokota T. Adherence characteristics to human small intestinal mucosa of Escherichia coli isolated from patients with diarrhea or urinary tract infections. J Infect Dis. 1990 Oct;162(4):896–908. doi: 10.1093/infdis/162.4.896. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]