Abstract
Opie suggested in 1901 that a common channel between the pancreatic duct and the common bile duct is created when a gall stone becomes impacted at the duodenal papilla. He proposed that bile would regurgitate into the pancreas and trigger pancreatitis. The case is reported of a 22 year old woman with an impacted stone at the duodenal papilla creating a common channel. The patient suffered from acute pancreatitis. Three days before the onset of pancreatitis, however, a T drain had been inserted into the common bile duct from which bile had been flowing freely and continuously. Moreover, amylase activity in fluid from the T drain was 49,000 U/l at the onset of pancreatitis pointing to reflux of pancreatic juice into the biliary tract. The amylase activity in bile decreased rapidly after endoscopic papillotomy and retrieval of the stone. The events participating in the development of acute gall stone induced pancreatitis in this patient with a common channel situation permitted reflux of pancreatic juice into the biliary tract rather than bile into the pancreas. Impairment of pancreatic outflow by a gall stone was probably the primary triggering event, rather than the regurgitation of bile into the pancreas. Preventive or therapeutic treatment in gall stone pancreatitis should be aimed at the urgent restoration of pancreatic flow rather than at the prevention of a hypothetical bile reflux.
Full text
PDF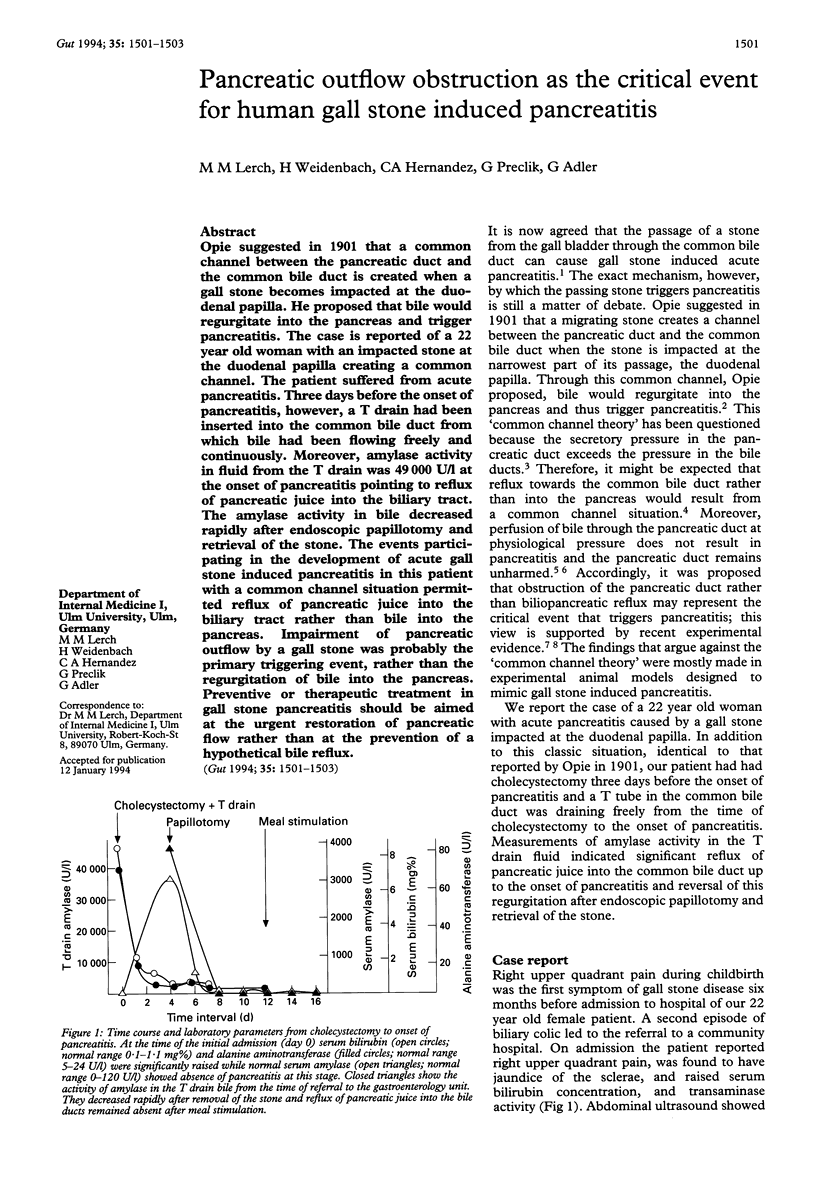


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta J. M., Ledesma C. L. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974 Feb 28;290(9):484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- Aho H. J., Nevalainen T. J. Experimental pancreatitis in the rat. Ultrastructure of sodium taurocholate-induced pancreatic lesions. Scand J Gastroenterol. 1980;15(4):417–424. doi: 10.3109/00365528009181494. [DOI] [PubMed] [Google Scholar]
- DiMagno E. P., Shorter R. G., Taylor W. F., Go V. L. Relationships between pancreaticobiliary ductal anatomy and pancreatic ductal and parenchymal histology. Cancer. 1982 Jan 15;49(2):361–368. doi: 10.1002/1097-0142(19820115)49:2<361::aid-cncr2820490225>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- ELLIOTT D. W., WILLIAMS R. D., ZOLLINGER R. M. Alterations in the pancreatic resistance to bile in the pathogenesis of acute pancreatitis. Ann Surg. 1957 Oct;146(4):669–682. doi: 10.1097/00000658-195710000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S. T., Lai E. C., Mok F. P., Lo C. M., Zheng S. S., Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993 Jan 28;328(4):228–232. doi: 10.1056/NEJM199301283280402. [DOI] [PubMed] [Google Scholar]
- Hernández C. A., Lerch M. M. Sphincter stenosis and gallstone migration through the biliary tract. Lancet. 1993 May 29;341(8857):1371–1373. doi: 10.1016/0140-6736(93)90942-a. [DOI] [PubMed] [Google Scholar]
- Kelly T. R., Wagner D. S. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery. 1988 Oct;104(4):600–605. [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Rünzi M., Dawra R., Saluja M., Steer M. L. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993 Mar;104(3):853–861. doi: 10.1016/0016-5085(93)91022-a. [DOI] [PubMed] [Google Scholar]
- MENGUY R. B., HALLENBECK G. A., BOLLMAN J. L., GRINDLAY J. H. Intraductal pressures and sphincteric resistance in canine pancreatic and biliary ducts after various stimuli. Surg Gynecol Obstet. 1958 Mar;106(3):306–320. [PubMed] [Google Scholar]
- Neoptolemos J. P., Carr-Locke D. L., London N. J., Bailey I. A., James D., Fossard D. P. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988 Oct 29;2(8618):979–983. doi: 10.1016/s0140-6736(88)90740-4. [DOI] [PubMed] [Google Scholar]
- ROBINSON T. M., DUNPHY J. E. Continuous perfusion of bile and protease activators through the pancreas. JAMA. 1963 Feb 16;183:530–533. doi: 10.1001/jama.1963.63700070004009a. [DOI] [PubMed] [Google Scholar]
- Rünzi M., Saluja A., Lerch M. M., Dawra R., Nishino H., Steer M. L. Early ductal decompression prevents the progression of biliary pancreatitis: an experimental study in the opossum. Gastroenterology. 1993 Jul;105(1):157–164. doi: 10.1016/0016-5085(93)90021-4. [DOI] [PubMed] [Google Scholar]
- STERLING J. A. The common channel for bile and pancreatic ducts. Surg Gynecol Obstet. 1954 Apr;98(4):420–424. [PubMed] [Google Scholar]
- Stone H. H., Fabian T. C., Dunlop W. E. Gallstone pancreatitis: biliary tract pathology in relation to time of operation. Ann Surg. 1981 Sep;194(3):305–312. doi: 10.1097/00000658-198109000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE T. T., MAGEE D. F. Perfusion of the dog pancreas with bile without production of pancreatitis. Ann Surg. 1960 Feb;151:245–250. doi: 10.1097/00000658-196002000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]



