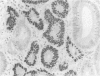Abstract
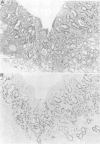
Abnormalities of the tumour suppressor gene p53 have been shown in approximately 60% of advanced gastric adenocarcinomas and it has been suggested that the immunohistochemical finding of increased p53 expression is a prognostic marker in gastric cancer. No studies of early (T1) tumours have been reported. Over expression of p53 protein in 95 early gastric carcinomas and in adjacent mucosa was investigated using immunohistochemistry with antibody CM1. Thirty five per cent of the tumours were positive. The frequency of p53 positivity in tumours of tubular histological type (46%) was significantly higher than that in signet ring tumours (10%) (p = 0.006), and neoplasms that invaded deeply into the submucosa were more frequently positive (45%) than others (30%). Five of eight (62%) T1 tumours with lymph node metastases showed immunoreactive p53. In signet ring tumours, immunopositivity correlated with the frequency of DNA aneuploidy. p53 Over expression was also found in 15% of 26 examples of high grade dysplasia in mucosa adjacent to invasive tumours. No positivity was found in intestinal metaplasia or in normal mucosa. The findings show that immunocytochemically demonstrable over expression of p53 correlates with other morphological markers of aggressiveness in T1 gastric adenocarcinoma. The increasing frequency of p53 immunoreactivity in the sequence of high grade dysplasia-->early gastric cancer-->advanced gastric cancer supports the view that abnormalities of p53 are related to tumour progression in gastric carcinogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bennett W. P., Hollstein M. C., He A., Zhu S. M., Resau J. H., Trump B. F., Metcalf R. A., Welsh J. A., Midgley C., Lane D. P. Archival analysis of p53 genetic and protein alterations in Chinese esophageal cancer. Oncogene. 1991 Oct;6(10):1779–1784. [PubMed] [Google Scholar]
- Brito M. J., Filipe M. I., Williams G. T., Thompson H., Ormerod M. G., Titley J. DNA ploidy in early gastric carcinoma (T1): a flow cytometric study of 100 European cases. Gut. 1993 Feb;34(2):230–234. doi: 10.1136/gut.34.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Vojtesek B., Stasková Z., Rejthar A., Kovarík J., Lane D. P. Patterns of expression of the p53 tumour suppressor in human breast tissues and tumours in situ and in vitro. Int J Cancer. 1990 Nov 15;46(5):839–844. doi: 10.1002/ijc.2910460515. [DOI] [PubMed] [Google Scholar]
- Carder P., Wyllie A. H., Purdie C. A., Morris R. G., White S., Piris J., Bird C. C. Stabilised p53 facilitates aneuploid clonal divergence in colorectal cancer. Oncogene. 1993 May;8(5):1397–1401. [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Joypaul B. V., Newman E. L., Hopwood D., Grant A., Qureshi S., Lane D. P., Cuschieri A. Expression of p53 protein in normal, dysplastic, and malignant gastric mucosa: an immunohistochemical study. J Pathol. 1993 Jul;170(3):279–283. doi: 10.1002/path.1711700310. [DOI] [PubMed] [Google Scholar]
- Kodama Y., Inokuchi K., Soejima K., Matsusaka T., Okamura T. Growth patterns and prognosis in early gastric carcinoma. Superficially spreading and penetrating growth types. Cancer. 1983 Jan 15;51(2):320–326. doi: 10.1002/1097-0142(19830115)51:2<320::aid-cncr2820510226>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Matozaki T., Sakamoto C., Matsuda K., Suzuki T., Konda Y., Nakano O., Wada K., Uchida T., Nishisaki H., Nagao M. Missense mutations and a deletion of the p53 gene in human gastric cancer. Biochem Biophys Res Commun. 1992 Jan 15;182(1):215–223. doi: 10.1016/s0006-291x(05)80133-0. [DOI] [PubMed] [Google Scholar]
- Morson B. C., Sobin L. H., Grundmann E., Johansen A., Nagayo T., Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol. 1980 Aug;33(8):711–721. doi: 10.1136/jcp.33.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T., Tsujino T., Yoshida K., Nakayama H., Haruma K., Ito H., Nakamura Y., Kajiyama G., Tahara E. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res. 1991 Jun 1;51(11):2926–2931. [PubMed] [Google Scholar]
- Wright P. A., Williams G. T. Molecular biology and gastric carcinoma. Gut. 1993 Feb;34(2):145–147. doi: 10.1136/gut.34.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992 Apr;166(4):329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]