Abstract
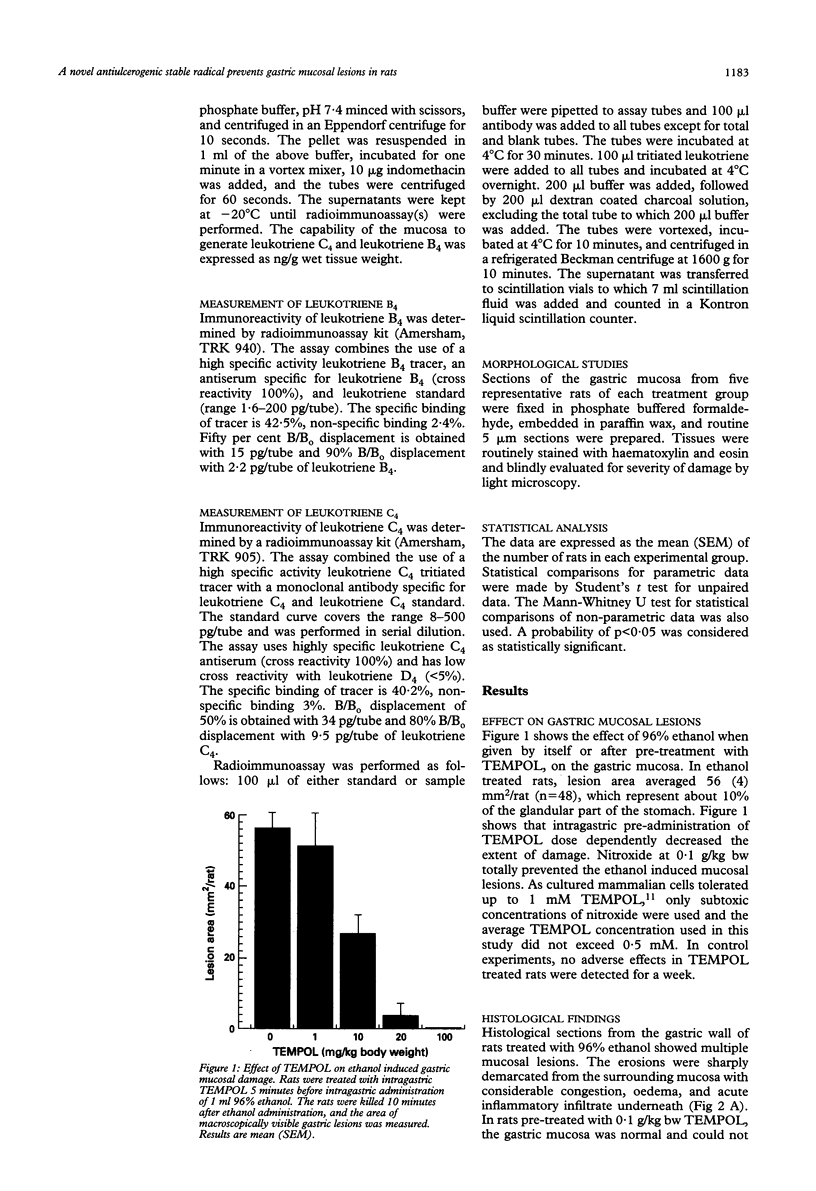
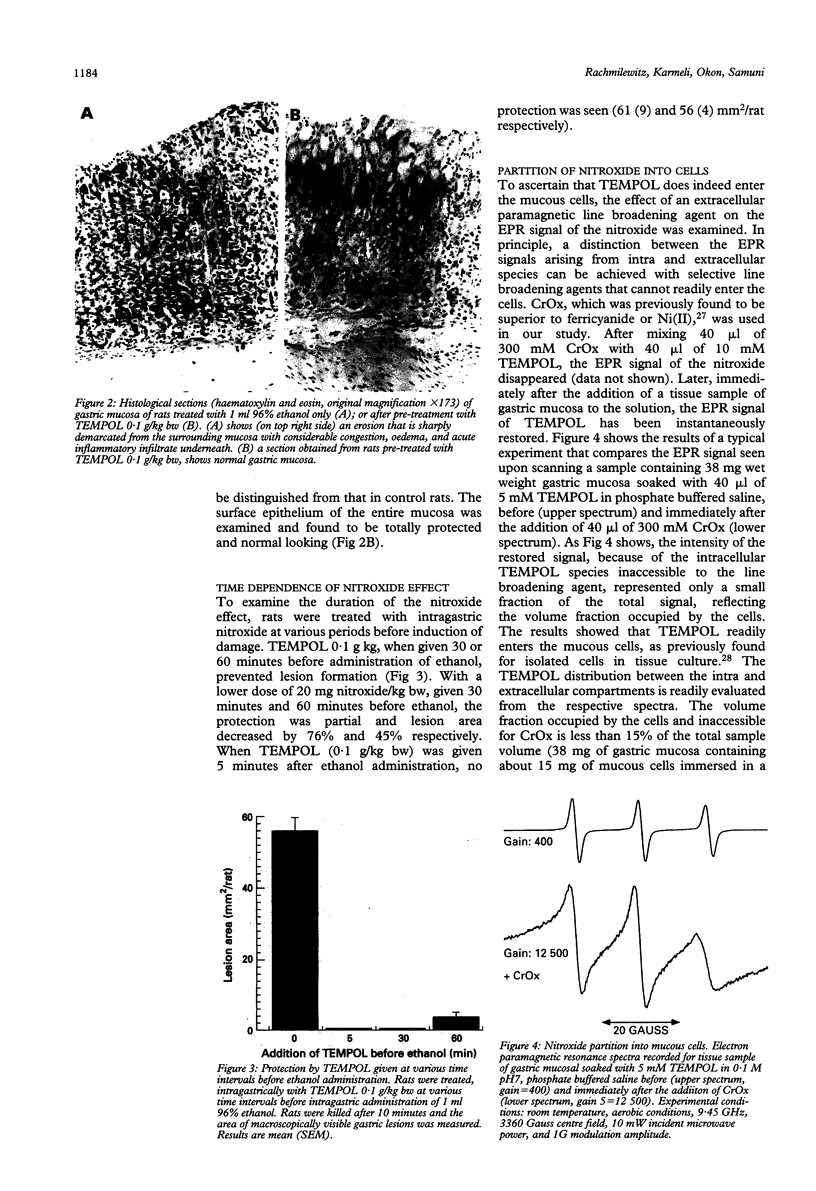
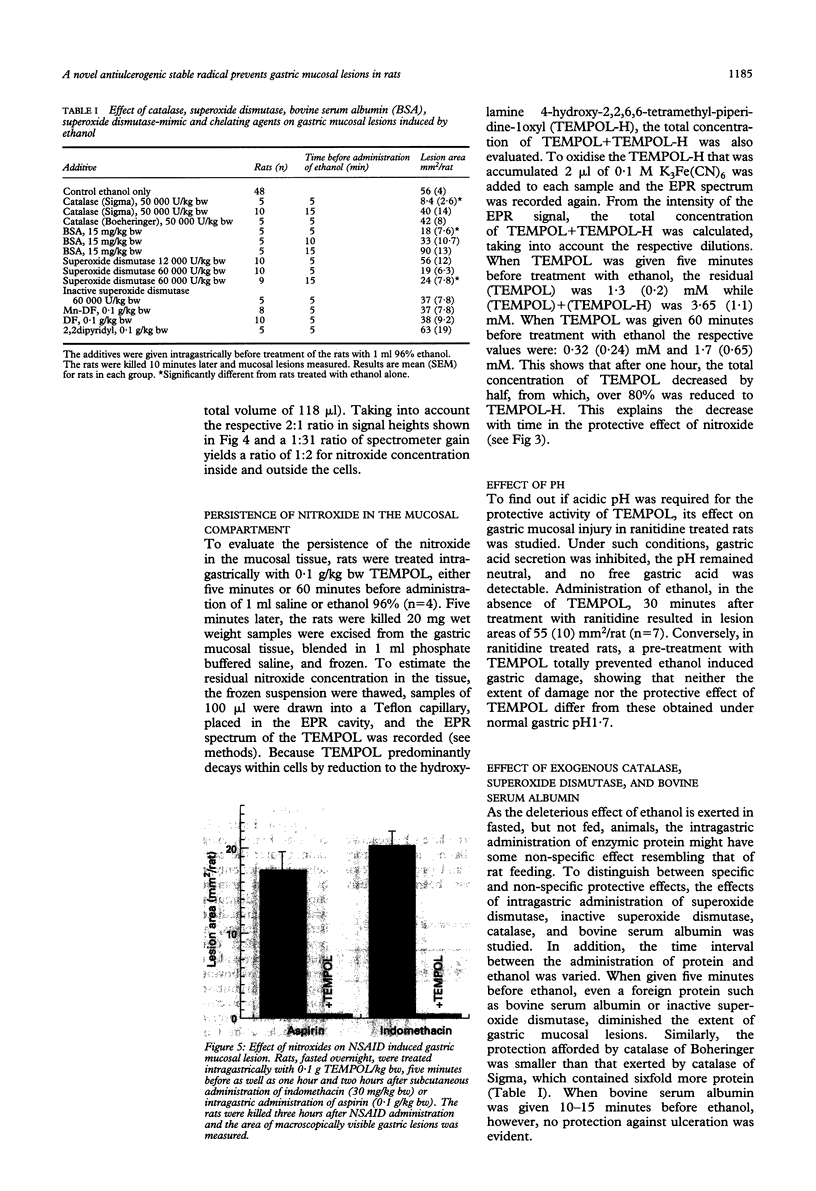
The pathogenesis of gastric mucosal injury is still poorly understood. Recent reports implicate redox active metals and reactive oxygen species as mediators of gastric damage induced by ethanol or non-steroidal anti-inflammatory drugs. Attempts were made therefore to prevent gastric injury using chelators and the antioxidant enzymes catalase and superoxide dismutase. These attempts, at best, would only detoxify extracellular reactive species, such as those produced by activated circulating granulocytes and macrophages. This study utilises another strategy by pre-emption of both intra and extracellular reactive species using radical-radical annihilation reactions and by detoxifying redox active transition metals. Nitroxide, stable free radicals were shown to enter mucous cells, protect against the ethanol induced damage, and prevent gastric lesions induced by aspirin, indomethacin, 25% NaCl, or 0.6 N HCl. These findings confirm that gastric mucosal damage from the above agents is mediated by free radicals and, moreover, introduce a prototypical agent within a potential new class of gastric ulcer preventing drugs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankel E. G., Lai C. S., Hopwood L. E., Zivkovic Z. Cytotoxicity of commonly used nitroxide radical spin probes. Life Sci. 1987 Feb 2;40(5):495–498. doi: 10.1016/0024-3205(87)90116-0. [DOI] [PubMed] [Google Scholar]
- Bacic G., Nilges M. J., Magin R. L., Walczak T., Swartz H. M. In vivo localized ESR spectroscopy reflecting metabolism. Magn Reson Med. 1989 May;10(2):266–272. doi: 10.1002/mrm.1910100211. [DOI] [PubMed] [Google Scholar]
- Belkin S., Mehlhorn R. J., Packer L. Electron spin resonance oximetry. Methods Enzymol. 1988;167:670–677. doi: 10.1016/0076-6879(88)67077-7. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Froncisz W., Lai C. S., Hyde J. S. Spin-label oximetry: kinetic study of cell respiration using a rapid-passage T1-sensitive electron spin resonance display. Proc Natl Acad Sci U S A. 1985 Jan;82(2):411–415. doi: 10.1073/pnas.82.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvan D., Saltman P., Powell S. R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4680–4684. doi: 10.1073/pnas.88.11.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockner J. F., Chan H. C., Swartz H. M. In vivo oximetry using a nitroxide-liposome system. Magn Reson Med. 1991 Jul;20(1):123–133. doi: 10.1002/mrm.1910200113. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Hahn S. M., Krishna C. M., Samuni A., Mitchell J. B., Russo A. Mn(III)-desferrioxamine superoxide dismutase-mimic: alternative modes of action. Arch Biochem Biophys. 1991 Jul;288(1):215–219. doi: 10.1016/0003-9861(91)90186-m. [DOI] [PubMed] [Google Scholar]
- Hahn S. M., Tochner Z., Krishna C. M., Glass J., Wilson L., Samuni A., Sprague M., Venzon D., Glatstein E., Mitchell J. B. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992 Apr 1;52(7):1750–1753. [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7(6):645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Mutoh H., Razandi M., Sugimoto T., Ivey K. J. Role for iron in reactive oxygen species-mediated cytotoxicity to cultured rat gastric mucosal cells. Am J Physiol. 1991 Apr;260(4 Pt 1):G556–G563. doi: 10.1152/ajpgi.1991.260.4.G556. [DOI] [PubMed] [Google Scholar]
- Karmeli F., Eliakim R., Okon E., Rachmilewitz D. Gastric mucosal damage by ethanol is mediated by substance P and prevented by ketotifen, a mast cell stabilizer. Gastroenterology. 1991 May;100(5 Pt 1):1206–1216. [PubMed] [Google Scholar]
- Keana J. F., Pou S., Rosen G. M. Nitroxides as potential contrast enhancing agents for MRI application: influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn Reson Med. 1987 Dec;5(6):525–536. doi: 10.1002/mrm.1910050603. [DOI] [PubMed] [Google Scholar]
- Krishna M. C., DeGraff W., Tamura S., Gonzalez F. J., Samuni A., Russo A., Mitchell J. B. Mechanisms of hypoxic and aerobic cytotoxicity of mitomycin C in Chinese hamster V79 cells. Cancer Res. 1991 Dec 15;51(24):6622–6628. [PubMed] [Google Scholar]
- Krishna M. C., Grahame D. A., Samuni A., Mitchell J. B., Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvietys P. R., Twohig B., Danzell J., Specian R. D. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 1990 Apr;98(4):909–920. doi: 10.1016/0016-5085(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Lai C. S., Froncisz W., Hopwood L. E. An evaluation of paramagnetic broadening agents for spin probe studies of intact mammalian cells. Biophys J. 1987 Oct;52(4):625–628. doi: 10.1016/S0006-3495(87)83253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. B., DeGraff W., Kaufman D., Krishna M. C., Samuni A., Finkelstein E., Ahn M. S., Hahn S. M., Gamson J., Russo A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch Biochem Biophys. 1991 Aug 15;289(1):62–70. doi: 10.1016/0003-9861(91)90442-l. [DOI] [PubMed] [Google Scholar]
- Mitchell J. B., Samuni A., Krishna M. C., DeGraff W. G., Ahn M. S., Samuni U., Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990 Mar 20;29(11):2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- Mutoh H., Hiraishi H., Ota S., Ivey K. J., Terano A., Sugimoto T. Role of oxygen radicals in ethanol-induced damage to cultured gastric mucosal cells. Am J Physiol. 1990 Apr;258(4 Pt 1):G603–G609. doi: 10.1152/ajpgi.1990.258.4.G603. [DOI] [PubMed] [Google Scholar]
- Nilsson U. A., Olsson L. I., Carlin G., Bylund-Fellenius A. C. Inhibition of lipid peroxidation by spin labels. Relationships between structure and function. J Biol Chem. 1989 Jul 5;264(19):11131–11135. [PubMed] [Google Scholar]
- Nordmann R., Ribière C., Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12(3):219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Otamiri T., Sjödahl R. Oxygen radicals: their role in selected gastrointestinal disorders. Dig Dis. 1991;9(3):133–141. doi: 10.1159/000171299. [DOI] [PubMed] [Google Scholar]
- Pihan G., Regillo C., Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987 Dec;32(12):1395–1401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- Rabinowitch H. D., Rosen G. M., Fridovich I. A mimic of superoxide dismutase activity protects Chlorella sorokiniana against the toxicity of sulfite. Free Radic Biol Med. 1989;6(1):45–48. doi: 10.1016/0891-5849(89)90158-5. [DOI] [PubMed] [Google Scholar]
- Salim A. S. Gastric mucosal cytoprotection in the rat by scavenging oxygen-derived free radicals. Am J Med Sci. 1991 Nov;302(5):287–291. doi: 10.1097/00000441-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Salim A. S. Removing oxygen-derived free radicals stimulates healing of ethanol-induced erosive gastritis in the rat. Digestion. 1990;47(1):24–28. doi: 10.1159/000200472. [DOI] [PubMed] [Google Scholar]
- Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur J Biochem. 1983 Dec 1;137(1-2):119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Samuni A., Carmichael A. J., Russo A., Mitchell J. B., Riesz P. On the spin trapping and ESR detection of oxygen-derived radicals generated inside cells. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7593–7597. doi: 10.1073/pnas.83.20.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A., Chevion M., Czapski G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J Biol Chem. 1981 Dec 25;256(24):12632–12635. [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988 Dec 5;263(34):17921–17924. [PubMed] [Google Scholar]
- Samuni A., Winkelsberg D., Pinson A., Hahn S. M., Mitchell J. B., Russo A. Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J Clin Invest. 1991 May;87(5):1526–1530. doi: 10.1172/JCI115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Sutherland L. R. 5-Aminosalicylates for prevention of recurrence in patients with Crohn's disease: time for a reappraisal? J Clin Gastroenterol. 1991 Feb;13(1):5–7. doi: 10.1097/00004836-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Szelenyi I., Brune K. Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig Dis Sci. 1988 Jul;33(7):865–871. doi: 10.1007/BF01550977. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Ueshima K., Hironaka Y., Fujioka Y., Matsumoto J., Okabe S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion. 1991;49(3):175–184. doi: 10.1159/000200718. [DOI] [PubMed] [Google Scholar]
- Terano A., Hiraishi H., Ota S., Shiga J., Sugimoto T. Role of superoxide and hydroxyl radicals in rat gastric mucosal injury induced by ethanol. Gastroenterol Jpn. 1989 Oct;24(5):488–493. doi: 10.1007/BF02773874. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Naito Y., Kishi A., Tomii T., Kaneko T., Iinuma S., Ichikawa H., Yasuda M., Takahashi S., Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993 Jun;34(6):732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]



