Abstract
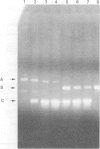
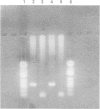
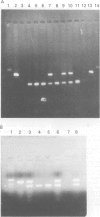
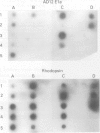
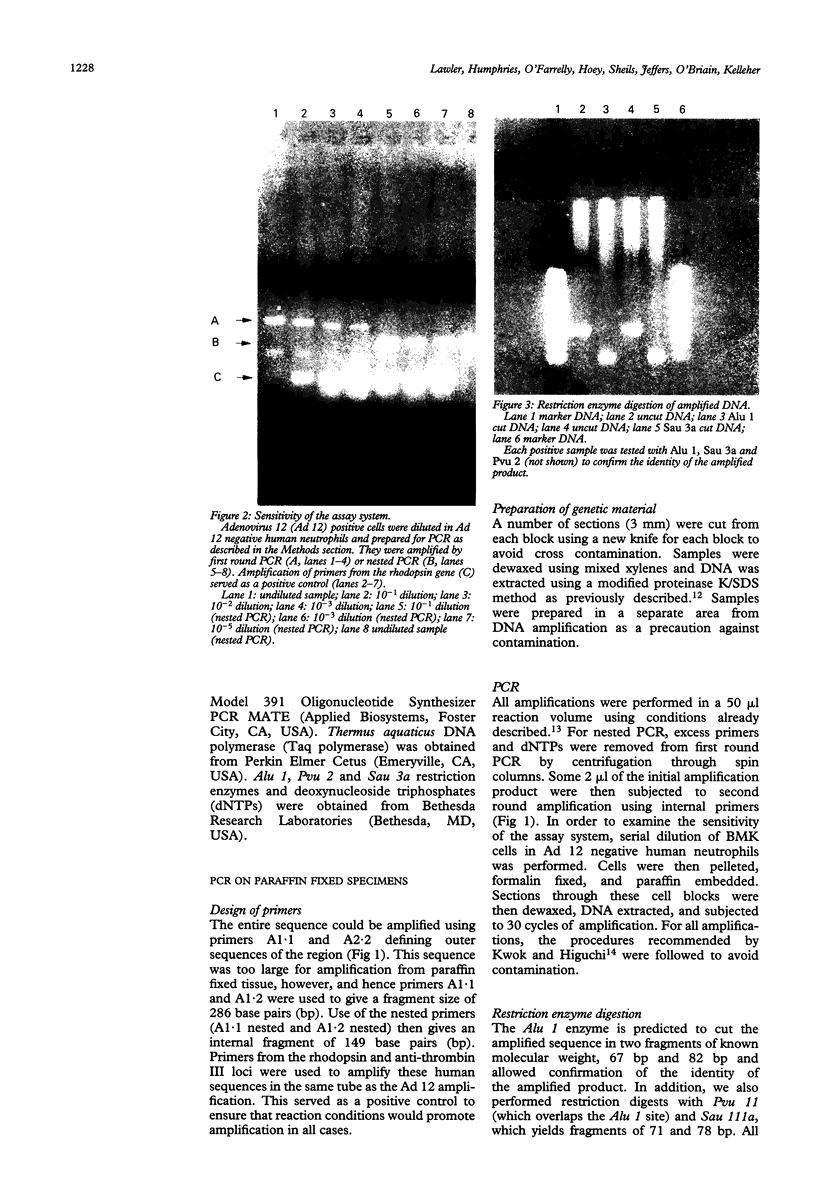
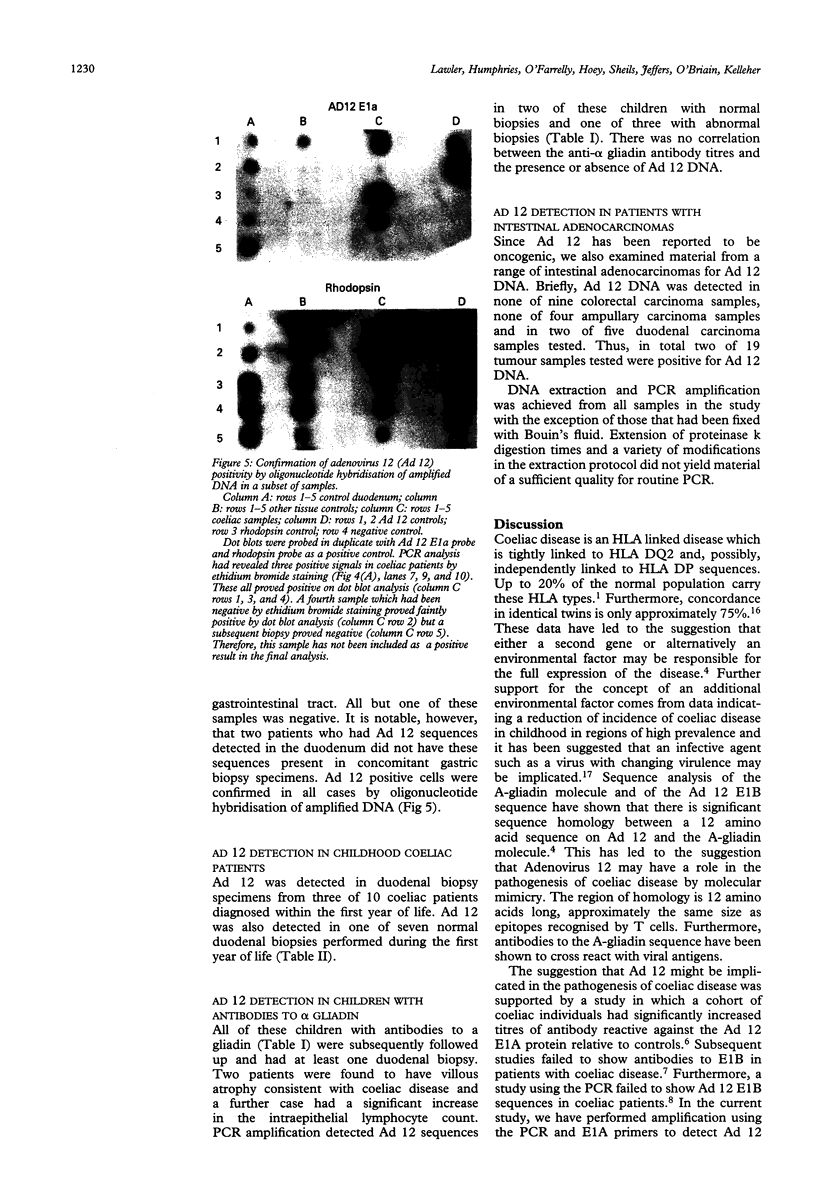
A 12 amino acid sequence from the adenovirus 12 E1B protein is homologous at the protein level with a similar 12-mer derived from the wheat protein A-gliadin. It has been suggested that exposure to Ad 12 could sensitise individuals to gliadins with resultant gluten sensitive enteropathy. In this study, the polymerase chain reaction (PCR) was used to analyse duodenal biopsy tissue from patients with coeliac disease for the presence of Ad 12. The sensitivity of the assay system was at least 1 in 10(5) cells and specificity was confirmed both by probing with an internal oligonucleotide and by direct sequencing. Ad 12 sequences were detected in three of 17 patients with adult coeliac disease and in five of 16 adult controls with normal duodenal biopsies. Since exposure to the virus would be predicted to occur in infancy we also studied patients with childhood coeliac disease diagnosed at less than 1 year of age. Ad 12 was positive in three of 10 childhood coeliac patients and one of seven controls. In addition, we studied a cohort of patients who presented with a diarrhoeal illness and associated anti alpha gliadin antibodies in 1983. These patients had duodenal biopsies performed at this time. One of three patients with abnormal histology had detectable Ad 12 while two of 14 with normal findings were positive for Ad 12. Finally, the potential oncogenic nature of Ad 12 prompted examination of a group of patients with intestinal tumours. Ad 12 DNA was, however, in only two of 19 tumour samples tested. These data indicate that Ad 12 can be successfully detected using PCR on paraffin embedded tissue. Furthermore, Ad 12 was detected at a relatively high level in normal duodenum. The results do not, however, support the hypothesis that prior exposure to Ad 12 is implicated in the pathogenesis of coeliac disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Childhood coeliac disease is disappearing. Lancet. 1980 Dec 20;2(8208-8209):1359–1360. [PubMed] [Google Scholar]
- Cooper B. T., Holmes G. K., Ferguson R., Cooke W. T. Celiac disease and malignancy. Medicine (Baltimore) 1980 Jul;59(4):249–261. doi: 10.1097/00005792-198007000-00002. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Harlow E. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 1992;12:161–195. [PubMed] [Google Scholar]
- Green M., Wold W. S., Mackey J. K., Rigden P. Analysis of human tonsil and cancer DNAs and RNAs for DNA sequences of group C (serotypes 1, 2, 5, and 6) human adenoviruses. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6606–6610. doi: 10.1073/pnas.76.12.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdle P. D., Blair Zajdel M. E., Smart C. J., Trejdosiewicz L. K., Blair G. E., Losowky M. S. Lack of a serologic response to an E1B protein of adenovirus 12 in coeliac disease. Scand J Gastroenterol. 1989 Apr;24(3):282–286. doi: 10.3109/00365528909093047. [DOI] [PubMed] [Google Scholar]
- Jackson D. P., Lewis F. A., Taylor G. R., Boylston A. W., Quirke P. Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. J Clin Pathol. 1990 Jun;43(6):499–504. doi: 10.1136/jcp.43.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Hubert J. J., Bernardin J. E., Kasarda D. D. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med. 1984 Nov 1;160(5):1544–1557. doi: 10.1084/jem.160.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Harwood J. I., Bugawan T. L., Erlich H. A. Structural analysis of the HLA-DR, -DQ, and -DP alleles on the celiac disease-associated HLA-DR3 (DRw17) haplotype. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6274–6278. doi: 10.1073/pnas.86.16.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Paterson Y. J., Kumar P. J., Kasarda D. D., Carbone F. R., Unsworth D. J., Austin R. K. Evidence for the role of a human intestinal adenovirus in the pathogenesis of coeliac disease. Gut. 1987 Aug;28(8):995–1001. doi: 10.1136/gut.28.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Hinrichs S. H., Isselbacher K. J., Jay G. Transgenic mouse model for human gastric carcinoma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5615–5619. doi: 10.1073/pnas.86.14.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lawler M., McCann S. R., Conneally E., Humphries P. Chimaerism following allogeneic bone marrow transplantation: detection of residual host cells using the polymerase chain reaction. Br J Haematol. 1989 Oct;73(2):205–210. doi: 10.1111/j.1365-2141.1989.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Mahon J., Blair G. E., Wood G. M., Scott B. B., Losowsky M. S., Howdle P. D. Is persistent adenovirus 12 infection involved in coeliac disease? A search for viral DNA using the polymerase chain reaction. Gut. 1991 Oct;32(10):1114–1116. doi: 10.1136/gut.32.10.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrelly C., Feighery C., O'Briain D. S., Stevens F., Connolly C. E., McCarthy C., Weir D. G. Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma. Br Med J (Clin Res Ed) 1986 Oct 11;293(6552):908–910. doi: 10.1136/bmj.293.6552.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Markussen G., Ek J., Gjerde H., Vartdal F., Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989 Jan 1;169(1):345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., Israel A., Kourilsky P., van der Eb A. J. Adenovirus E1A-mediated regulation of class I MHC expression. EMBO J. 1986 Feb;5(2):335–341. doi: 10.1002/j.1460-2075.1986.tb04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesy C. J., Greenson J. K., Papp A. C., Snyder P. J., Qualman S. J., Prior T. W. Evaluation of celiac disease biopsies for adenovirus 12 DNA using a multiplex polymerase chain reaction. Mod Pathol. 1993 Jan;6(1):61–64. [PubMed] [Google Scholar]
- Walker-Smith J. A. Discordance for childhood coeliac disease in monozygotic twins. Gut. 1973 May;14(5):374–375. doi: 10.1136/gut.14.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]