Abstract
The C-type lectin dendritic cell-specific ICAM 3-grabbing nonintegrin (DC-SIGN)/CD209 efficiently binds several pathogens, including HIV-1. DC-SIGN is expressed on monocyte-derived DCs in culture, and importantly, it is able to sequester HIV-1 within cells and facilitate transmission of virus to CD4+ T cells. To investigate DC-SIGN function, we have generated new mAbs. We report in this study that these and prior anti-DC-SIGN mAbs primarily label macrophages in the medullary sinuses of noninflamed human lymph node. In contrast, expression is not detected on most DCs in the T cell area, except for occasional cells. We also noted that IL-4 alone can induce expression of DC-SIGN in CD14+ monocytes and circulating blood DCs. However, blockade of DC-SIGN with Abs and DC-SIGN small interfering RNA did not result in a major reduction in the capacity of these DCs to transfer HIV to T cells, confirming significant DC-SIGN-independent mechanisms. The blocking approaches did reduce HIV-1 transmission by DC-SIGN-transfected cells by >90%. DC-SIGN blockade also did not reduce the ability of DCs to stimulate T cell proliferation in the MLR. These results indicate that DC-SIGN has the potential to contribute to macrophage function in normal human lymph node, and that DCs do not require DC-SIGN to transmit HIV or to initiate T cell responses.
Dendritic cell-specific ICAM 3-grabbing nonintegrin (DC-SIGN)3/CD209 is a C-type lectin expressed on DCs differentiated in vitro from CD14+ cells cultured with IL-4 and GM-CSF (1). Importantly, several microbial agents bind to DC-SIGN, including viruses, bacteria, parasites, and yeast (review in Ref. 2). This field began with the discovery that DC-SIGN binds the HIV-1 gp120 envelope protein with high affinity (3, 4). After uptake into monocyte-derived DCs and into DC-SIGN transfectants, HIV-1 remains infectious for some time (5, 6) and is transmitted to T cells at contact zones termed virological synapses (7, 8). Binding of HIV-1 to DC-SIGN also can enhance direct HIV-1 infection in cis (6, 9), but much of the research has focused on the role of DC-SIGN as a receptor to explain the effective sequestration and transmission of HIV-1 from DCs to T cells in trans (4, 10-13). Nonetheless, the contribution of CD209 to HIV-1 transmission has been documented primarily with Raji cells transfected with DC-SIGN (4-6, 14). More recently DC-SIGN expression has been nullified in DCs derived from CD34+ progenitor cells with small interfering RNA (siRNA), and this resulted in a reduction of transmission of X4 tropic HIV in culture (15). For monocyte-derived DCs, some studies have reported that HIV-1 transmission from DCs to T cells is mediated exclusively by DC-SIGN (4, 5), whereas others report a relatively minor contribution of DC-SIGN (6, 14, 16-18). Thus, the need for DC-SIGN in HIV-1 transmission by monocyte-derived DCs is not clear.
In vivo expression of human DC-SIGN has been reported in cells, possibly DCs, tonsil, the dermis of skin, and the subepithelial region of cervix (1, 19-22). Studies of lymph nodes have been limited, but have stressed the presence of scattered DC-SIGN-bearing cells in the outer cortex, particularly in the subcapsular sinuses and perifollicular regions (1, 19, 23, 24). In this paper we have studied the in vivo expression and in vitro function of CD209. To facilitate our studies, we first prepared a new panel of mAbs using a recombinant vaccinia-DC-SIGN virus as an immunogen and then compared these mAbs with existing reagents. We report that CD209 in normal human lymph node is actually abundantly expressed by macrophages in the lymph node medulla, rather than DCs in the T cell area. Also, when we studied the blocking effects of anti-CD209 Abs and siRNA in monocyte-derived DCs or DCs from blood, our experiments did not reveal a major contribution of DC SIGN to HIV-1 transmission by DCs, in contrast to DC-SIGN transfectants or to the stimulating function of DCs in culture.
Materials and Methods
DC isolation
DCs were generated from the blood of normal donors, usually from buffy coats purchased from the New York Blood Center. Monocyte-derived DCs were prepared from PBMC as previously described (25, 26) with some modification. Briefly, CD14+ cells were obtained using anti-CD14 beads (Miltenyi Biotec) and cultured for 6 days with IL-4 (R&D Systems; 10 ng/ml) and GM-CSF (Immunex; 100 IU/ml). The culture medium was RPMI 1640 supplemented with 5% AB human serum (Gemini Bio-Products). Myeloid DCs were also isolated directly from Ficoll-Hypaque-enriched total blood mononuclear cells using the BDCA-1 isolation kit (Miltenyi Biotec). BDCA1-positive cells represent 0.5–2% of the PBMCs. The DCs were routinely phenotyped to determine contamination with CD3-, CD19-, and CD16-expressing cells. The preparations contained >0.05% CD3+ T cells and only traces of CD19+ and CD16+ cells.
Mice and immunizations
BALB/c mice, 6–8 wk old, were purchased from Charles River Breeding Laboratories and were used within 8 wk. Four BALB/c mice were immunized i.m. with 100 μg of enhanced GFP plasmid N1 (BD Clontech) expressing the extracellular domain of DC-SIGN fused to GFP. Mice were boosted three times with the same amount of plasmid 2 wk apart and were bled to test for the level of specific Ab in sera. The sera were screened for binding to Madin-Darby canine kidney (MDCK) cells transiently transfected with pCAGGS expressing full-length DC-SIGN and DCs. Five days before fusion, an immunized mouse was given 100 μl of 107 PFU/ml DC-SIGN/recombinant vaccinia virus i.p. Recombinant vaccinia virus stocks expressing full-length DC-SIGN were generated, plaque purified, and propagated in CV-1 African green monkey cells as previously described (27). On the day of fusion, the mouse was bled, and the spleen was removed for fusion. Fusion of splenocytes and SP2–0 cells were performed by standard techniques previously described (28). On day 10, 100 μl of hybridoma supernatants were collected from each of 960 wells for screening.
Screening of clones
Screening of supernatants was performed by immunostaining of MDCK cells transiently transfected with DC-SIGN/pCAGGS mammalian expression vector. MDCK cells were transiently transfected in 96-well plates 24 h before screening using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen Life Technologies). The cells were fixed with 1% formaldehyde/PBS, washed, and blocked with 1% BSA/PBS for 15 min at room temperature. Supernatants from the wells were added to the MDCK monolayer for 1 h at room temperature, and binding was identified with anti-mouse IgG HRP-conjugated Ab, followed by the addition of the 3-amino-9-ethyl-carbazole substrate (Biomeda). The cells from the positive wells were cloned by limiting dilution to generate single-cell clones. Supernatants from the cloned wells were analyzed for binding to Raji cells expressing DC-SIGN and human DCs by flow cytometry using a Cytomics FC 500 (Beckman Coulter).
DC-SIGN-expressing cells
The generation of mammalian cells expressing type II transmembrane C-type lectins was described previously (29). In brief, the extracellular domains, a whole ectodomain, and a lectin domain of human DC-SIGN were expressed as soluble protein fused to the C terminus of murine IgG Fc. The cDNA constructs were inserted into the pCMV expression vector (BD Clontech) and transfected onto Chinese hamster ovary cells. Expression of the DC-SIGN domains was detected after cell permeabilization and FACS. Chinese hamster ovary cells expressing mouse DC-SIGN and SIGN-R1 have been described previously (30). Raji-DC-SIGN and Hep-LSIGN were provided by D. Littman (New York University, New York, NY) and J. McKeating (Rockefeller University, New York, NY), respectively. The expression of DC-SIGN and L-SIGN was monitored with specific mAbs. The anti-DC-SIGN Abs, 120507 (DC-SIGN specific), 120612 (reacting with DC-SIGN and L-SIGN), and 120604 (L-SIGN specific) were purchased from R&D Systems, whereas AZN-D1 (4) and MR-1 (31) were provided by Y. van Kooyk (Amsterdam, The Netherlands) and A. Corbi (Madrid, Spain), respectively.
Lymph node tissue section staining
Surgical specimens of noninflamed human lymph nodes from the chest and abdomen of cadaver transplant donors were provided by the New York Organ Donor Network. The seven nodes showed no evidence of follicular hyperplasia or infiltration with CD14+ monocytes or CD32+ granulocytes. Tissues were obtained in protocols approved by the institutional review boards of Weill Medical College, Cornell University, and Rockefeller University. Tissues were frozen and stored at −80°C. Cryostat sections (6 μm) were air dried and fixed in acetone for 10 min at room temperature. Ags were detected by immunofluorescence using anti-DC-SIGN mAbs (Table I), anti-DEC-205 (clone MG 38-2) (32), anti-CD68 (DakoCytomation), MMR (clone 3.29; provided by A. Lanzavecchia, Bellinzona, Switzerland), and anti-CD11c (BD Biosciences). All primary mAbs were used at 1 μg/ml. Appropriate anti-mouse Alexa 488 or Alexa 546-conjugated isotype-specific secondary Abs (Molecular Probes) were used at a dilution of 1/300 to reveal the primary mAbs. Sections were examined in a deconvolution microscope (AX70; Olympus).
Table I.
Specificity of the anti-DC-SIGN mAbsa
| DC-SIGN Abs Reactivity for: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immature |
Raji |
Hep |
Hep |
Human DC-SIGN |
||||||
| No. | Clone | Ig | Blot | DC | Raji | DC-SIGN | CD81 | L-SIGN | Lectin domain | Ectodomain |
| 1 | 9′E9A8 | IgG2a κ | No | Yes | No | Yes | No | No | Yes | Yes |
| 2 | 14E3G7 | IgG2b κ | Yes | Yes | No | Yes | No | Yes | No | Yes |
| 3 | 13C7F4 | IgG1κ | +/− | Yes | No | Yes | No | Yes | No | Yes |
| 4 | 19F7 | IgG2b κ | Yes | Yes | No | Yes | No | Yes | No | Yes |
Each Ab was assessed by FACS for their reactivity with DCs or transfected cells expressing DC-SIGN or L-SIGN. The clones were also tested for their ability to detect DC-SIGN in cell lysates by Western blot.
MLR
Immature or mature DCs were pretreated with anti-DC-SIGN Abs for 20 min at room temperature and then used to stimulate 105 T cells. The MLR was conducted in round-bottom, 96-well microtest trays in 0.2 ml of RPMI 1640/5% human serum in the continued presence of the blocking mAb at 20 μg/ml. Graded doses of DCs were added as indicated in Results. To monitor the MLR, the T cells were labeled with CFSE (Molecular Probes). Cells were stained with 2 μM CFSE for 10 min at 37°C, followed by quenching with FCS and three washes in complete medium. The MLR was assessed by CFSE dilution on days 4–6 (see Results),
Sorting of lentivirus-transduced immature DCs and Raji DC-SIGN transfectants
Immature DCs and Raji cells were transduced at a multiplicity of infection (MOI) of 20 with lentiviral vectors expressing siRNA for DC-SIGN (si DC-SIGN 11) or empty vector (15). After 48 h, cells were stained with anti-DC-SIGN-PE and sorted for low DC-SIGN-expressing cells as shown in Results. The cells were cultured for 24 h before use.
HIV-1 infection of immature DCs and Raji DC-SIGN transfectants
Cells were infected with the BaL isolate, which we grew in PHA-stimulated PBMCs. The virus was added at doses of 300–900 pg of p24 to 105 target cells for 2 h at 37°C to immature DCs differentiated from monocytes with GM-CSF and IL-4, DCs from fresh blood, or Raji cells stably transfected with DC-SIGN. To study the blocking capacity of anti-DC-SIGN, the indicated mAbs were added 20 min before infection and maintained through the 2-h infection period. The cells were washed four times. To determine the level of bound p24, cell lysates were obtained by adding 0.5% Triton. For studying HIV-1 transmission, 5 × 104 DC-SIGN-expressing cells were cultured with 105 activated T cells, and supernatants were collected and assayed for p24 by ELISA (Coulter).
Results
Generation of anti-DC-SIGN mAbs with recombinant vaccinia-DC-SIGN
To expand the availability of hybridomas secreting mAbs to DC-SIGN/CD209, we used monocyte-derived DCs to immunize mice. Although some investigators have succeeded in obtaining mAbs in this way (1, 31), our mice did not generate good Ab responses when tested with DC-SIGN transfectants even after four boosts. Next, we immunized mice twice with DC-SIGN DNA and subsequently boosted with vaccinia expressing DC-SIGN. The sera from immunized mice, but not the preimmune sera, were shown to react with immature monocyte-derived DCs by FACS. The immune spleen cells were fused with SP20 myeloma to generate hybridomas, which again were screened by FACS for reactivity on immature DCs. Twelve DC-SIGN-reactive mAbs were obtained and subcloned, spanning most of the major mouse Ig isotypes, and four were extensively characterized (Table I).
Each hybridoma was studied for the ability to stain DCs as well as DC-SIGN and L-SIGN transfectants (and nontransfected parental cells as controls) by flow cytometry (Fig. 1A). Only one clone recognized exclusively DC-SIGN (9E9A8, clone 1); all the others reacted with both DC-SIGN and L-SIGN transfectants. This is not surprising if one considers that DC-SIGN and L-SIGN are 73% homologous and are identical in the membrane-proximal repeat region of the ectodomain (33, 34). None of the Abs recognized mouse SIGNR-1 or mouse DC-SIGN transfectants (not shown). The hybridomas were examined for their capacity to detect DC-SIGN by Western blotting (Fig. 1B); five of the 12 mAbs blotted a major band at 50 kDa, corresponding to DC-SIGN, suggesting that they recognize conformation-independent determinants. The blots showed weaker bands at higher molecular masses which may represent aggregates of DC-SIGN. Our immunization procedure, DC-SIGN DNA prime, followed by vaccinia-DC SIGN boost, therefore elicits a good immune response. We proceeded to use our new anti-DC-SIGN reagents in parallel with other anti-DC-SIGN mAbs provided by colleagues (AZN-D1 (4) and MR-1 (31)) and commercial reagents purchased from R&D Systems.
FIGURE 1.
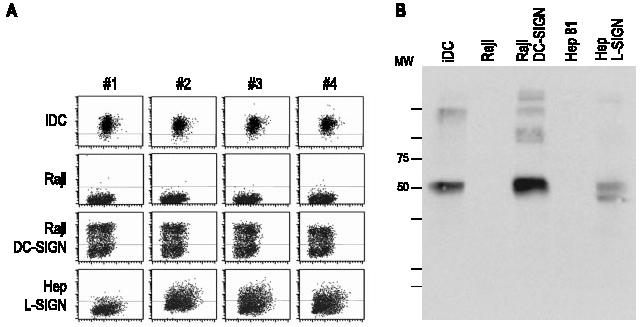
Reactivity of mAbs with DC-SIGN in transfected cells and DCs. A, Reactivity of anti-DC-SIGN mAb with DCs and transfectants expressing full-length DC-SIGN or L-SIGN. B, Reactivity by immunoblot of anti-DC-SIGN mAb 2. The indicated cells were lysates in RIPA buffer, equivalent to 3 × 105 cells. After separation on SDS-PAGE, the lysates were blotted with anti-DC-SIGN mAb 2.
DC-SIGN distribution in human lymph nodes
Although previous reports have identified scattered cells bearing DC-SIGN in the outer cortex of human lymph nodes (see Discussion), we surprisingly found that each of the mAbs to DC-SIGN in our large panel produced strong staining of large cells in the medullary sinuses (Fig. 2). Macrophages are known to be the predominant large cell in the medullary sinuses, and in fact, all cells that labeled for the macrophage markers, CD206 or macrophage mannose receptor and CD68, double labeled for DC-SIGN (Fig. 2). This was the case with all anti-DC-SIGN Abs tested, e.g., MR-1, AZN-D1, 507, and clone 1 from Table I (e.g., Fig. 2). To formally prove that the new anti-DC-SIGN mAb stained similarly to previous DC-SIGN Abs, we performed double labeling for MR-1 and found an overlap of the two labels (Fig. 3). Therefore, DC-SIGN is expressed abundantly in the macrophages of the medullary sinuses of uninflamed human lymph nodes.
FIGURE 2.
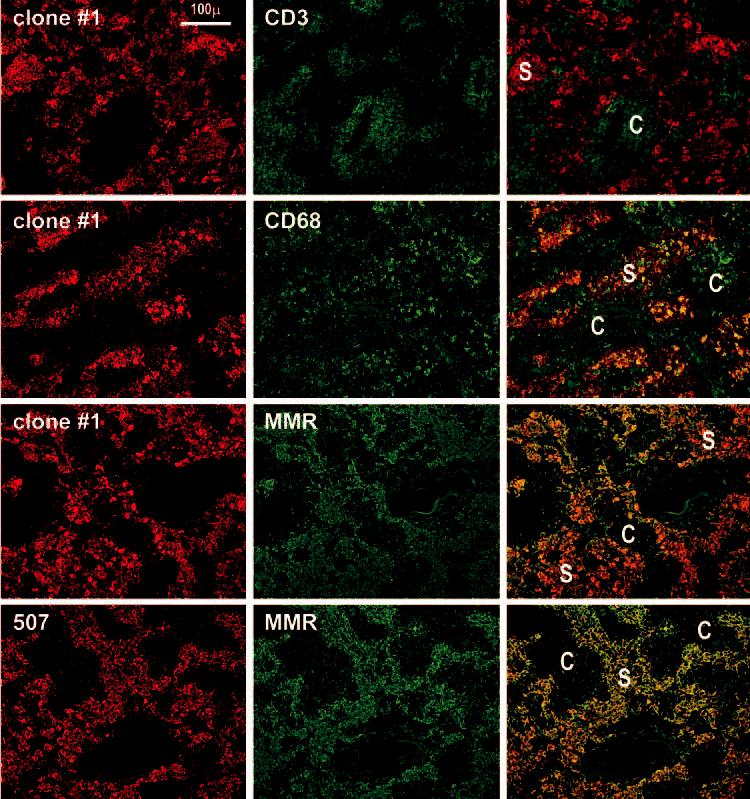
DC-SIGN staining is restricted to macrophages in the medullary sinuses (S) of lymph nodes. Two-color staining is shown, with red being the anti-DC-SIGN reagent, and green the other markers. First row, Clone 1 anti-DC-SIGN stains macrophages in the sinuses of the medulla and anti-CD3 stains T cells in the medullary cords (C). Second row, Clone 1 and anti-CD68 colabel medullary macrophages. Third row, Clone 1 and anti-macrophage mannose receptor (MMR) colabel macrophages in the lymph node medulla. Fourth row, Double staining using a commercial anti-DC-SIGN 507 and anti-mannose receptor mAbs.
FIGURE 3.
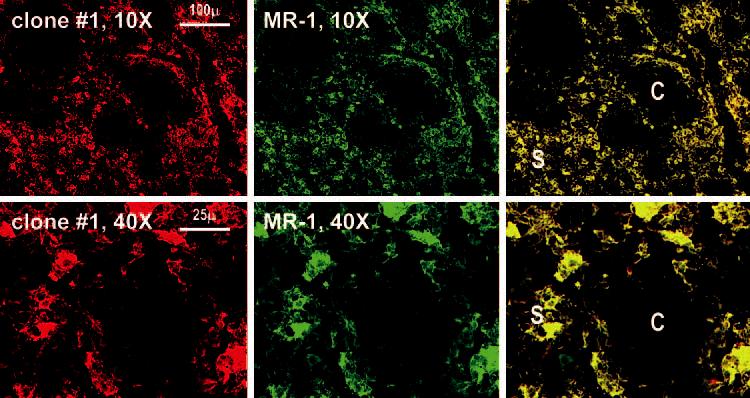
Identical staining by clone 1 anti-DC-SIGN and another established anti-DC-SIGN mAb. Lymph node medulla sections were double stained with our clone 1 and the anti-DC-SIGN mAb MR-1.
In the cortex, Abs to DC-SIGN did not show strong staining in either B cell or T cell areas (Fig. 4, top row). The B cell regions contained follicular DCs that stained with Abs to CD21 (Fig. 4, top row), whereas the T cell regions contained DCs that stained with Abs to DEC-205/CD205 and CD11c. Most DEC-205-positive DCs were DC-SIGN negative (Fig. 4, middle row). Likewise, DC-SIGN and CD11c primarily stained different cells: the former macrophages from the medulla, and the latter DCs in the cortex (Fig. 4, lower row, left). We verified these findings with mAbs that react with both DC-SIGN and L-SIGN. Again, these Abs did not stain CD11c+ cells in the cortex (Fig. 4, lower row, middle), but did stain medullary sinus macrophages (Fig. 4, lower row, right). In the medulla, there were cells that stained with mAbs to L-SIGN/DC-SIGN, but not with mAb to DC-SIGN only; these L-SIGN-positive cells presumably are sinusoidal endothelium (Fig. 4, lower row, right).
FIGURE 4.
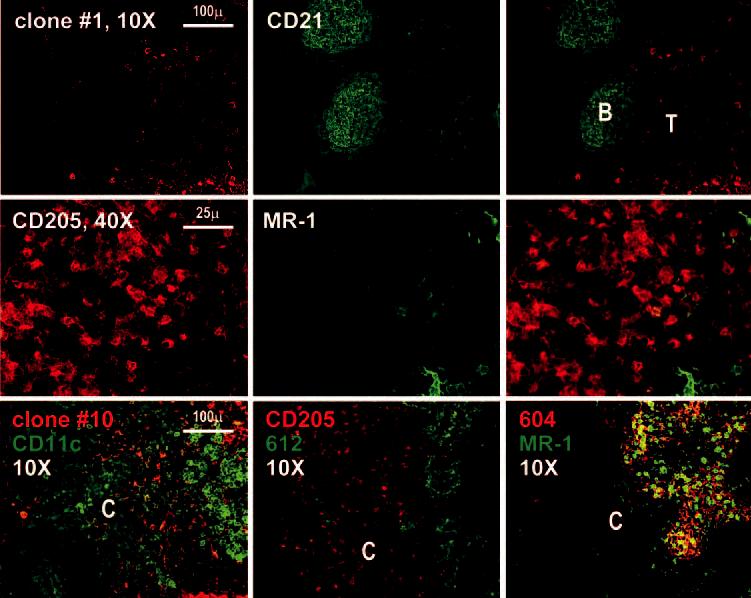
Immunostaining of the lymph node cortex with anti-DC-SIGN mAbs. First row, The lymph node cortex is shown at ×10 after staining with anti-CD21 (green) to define the B cell follicles (B) and the unstained T cell region (T). Second row, Images at higher magnification within the T cell region reveal that most DCs defined by staining with anti-CD205 are DC-SIGN negative. Third row, The left panel is a double staining with our clone 10 (specific for DC-SIGN and L-SIGN) and CD11c; the middle panel is a double staining with clone 612 (specific for DC SIGN and L-SIGN) and DEC205; the right panel shows a double staining using anti-L-SIGN specific mAb (604) with MR-1.
These data indicate that we could not readily identify cells in the lymph node with the markers found in monocyte-derived DCs, cells which have been the major object of research on DC-SIGN. However, in two of our lymph nodes, we were able to identify foci of cells with markers of monocyte-derived DCs, i.e., in addition to DC-SIGN, the cells expressed CD11c and DEC-205 (Fig. 5). These data indicate that DC-SIGN in the normal lymph node is primarily expressed on medullary macrophages, rather than the different types of DCs in the B and T cell areas, and that cells with the phenotype of monocyte-derived DCs are a small fraction of the DCs in lymph nodes.
FIGURE 5.
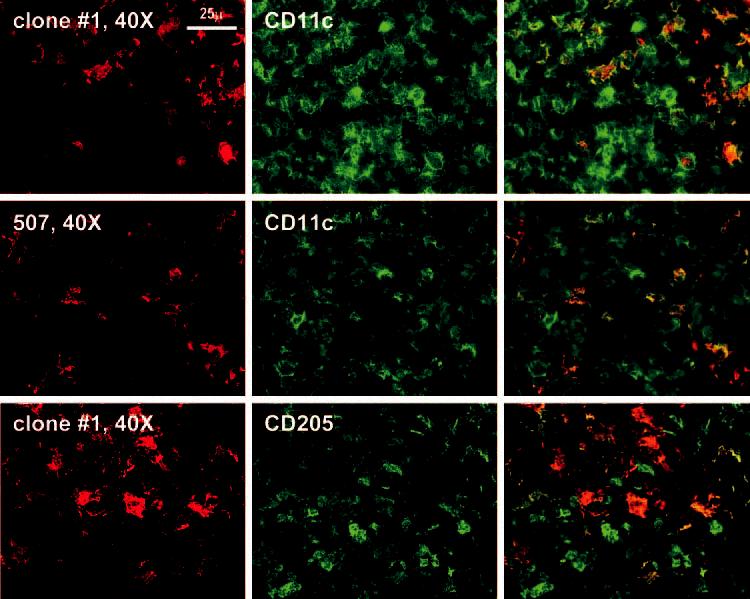
Immunostaining of the lymph node cortex. Higher magnification of the cortex where rare fields can be found in which red DC-SIGN-positive cells colabel with CD11c and anti-CD205.
DC-SIGN Abs inhibit HIV binding to Raji DC-SIGN transfectants, but not DCs
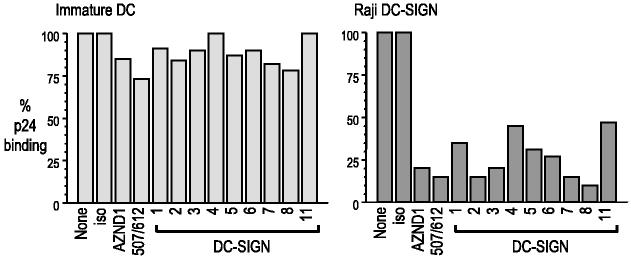
We then assessed a panel of anti-DC-SIGN mAbs for their ability to interfere with HIV-1 binding to Raji-DC-SIGN transfectants and monocyte-derived immature DCs. Cells were incubated with relatively low doses of virus (300–900 pg of p24/105 cells) for 2 h at 37°C, washed, and lysed in 0.5% Triton. The amount of HIV-1 gag p24 in the lysates was then quantified by ELISA. HIV-1 bound comparably to both types of cells in this assay (∼5–15 pg of p24), and the degree of binding was dependent on the viral load (not shown). In the case of the Raji transfectants, HIV-1 binding was markedly (90–95%) blocked by a mixture of two different commercially Abs (507 reacting with DC-SIGN and 612 reacting with DC-SIGN/DC-L-SIGN), AZN-D1, and the panel of our new Abs (Fig. 6, right panel). In contrast, these same anti-DC-SIGN mAbs only slightly decreased HIV-1 binding to DCs studied in parallel (Fig. 6, left panel). Therefore, HIV-1 binding to DC-SIGN transfectants is DC-SIGN dependent, but a DC-SIGN independent mechanism seems to predominate in monocyte-derived DCs, confirming previous reports with smaller numbers of mAb to DC-SIGN (6, 14, 16, 17).
FIGURE 6.
HIV-1 binding to DC-SIGN-expressing cells. Cells were incubated with anti-DC-SIGN mAbs or isotype control at 20 μg/ml for 20 min at room temperature and then infected with HIV-1 for 2 h at 37°C. Cells were washed and lysed in 0.5% Triton. The amount of p24 in the cell lysates was quantified by ELISA.
Effects of Abs and siRNA inhibition of DC-SIGN on transmission of HIV-1 from DC-SIGN transfectants and DCs to T cells
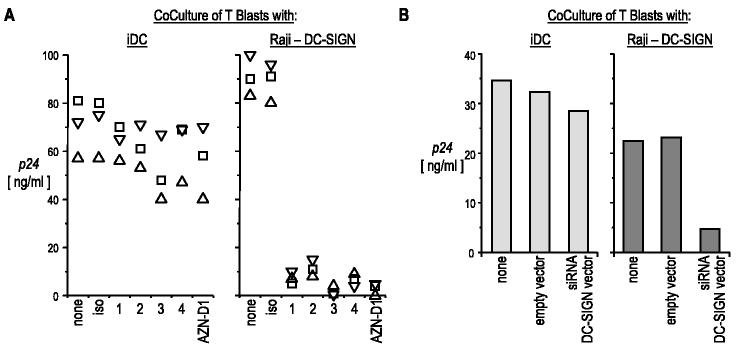
The anti-DC-SIGN mAbs were also tested for their capacity to inhibit HIV-1 transmission from HIV-1-infected cells to activated T cells. Cells preincubated with anti-DC-SIGN mAbs were exposed to virus for 2 h and, after extensive washes, were cocultured with T cells. Transfer of a productive infection to T cells was assessed in the culture supernatants by p24 ELISA. HIV-1 transmission from Raji-DC-SIGN transfectants to T cells was completely blocked by the many different anti-DC-SIGN and anti-L-SIGN Abs tested (Fig. 7A, right panel). However, the anti-DC-SIGN mAbs even at high concentrations (20 μg/ml) had only a minimal effect on HIV-1 transfer from monocyte-derived DC to T cells (Fig. 7A, left panel).
FIGURE 7.
Effect of anti-DC-SIGN mAbs on HIV-1 transmission to T blasts. A, The indicated cells (left panel, immature DCs; right panel, Raji-DC-SIGN) were incubated with anti-DC-SIGN mAbs as described in Fig. 6, and then infected with HIV-1 BaL in the continued presence of mAb. The cells were washed four times, and 5 × 104 infected cells were cocultured with 105 T blasts. After 5 days, p24 secreted in the culture supernatants was quantified by ELISA. Each symbol corresponds to an individual experiment. B, DC-SIGN-expressing cells were transduced with siRNA lentiviral vector or empty vector at an MOI of 20, sorted for low DC-SIGN expression, and infected with HIV-1 before addition to T cells. The resulting cells were cocultured and assayed as described above.
The siRNA treatment via lentiviral vectors has recently been used to down-regulate DC-SIGN expression in DCs derived from CD34-positive progenitors (15). We repeated this approach with Raji DC-SIGN transfectants and monocyte-derived DCs. Transfer of HIV-1 was markedly reduced when Raji-DC-SIGN transfectants were transduced with DC-SIGN siRNA vector, but not with a control lentiviral vector (Fig. 7B, right). For monocyte-derived DCs, DC-SIGN expression was also reduced, and we FACS-sorted the fraction of cells in which DC-SIGN expression was comparable to the isotype control mAb. In contrast to Raji DC-SIGN, immature monocyte-derived DCs, when suppressed with the DC-SIGN siRNA vector, were only marginally impaired in their capacity to transfer HIV-1 infection to T cells (Fig. 7B). These data confirm that monocyte-derived DCs have a major alternative pathway to DC-SIGN for transferring HIV-1 to competent T cells.
Induction of DC-SIGN on blood DC subsets does not affect HIV transmission
We compared the expression of DC-SIGN on monocyte-derived DCs, obtained in a standard way by culture with GM-CSF and IL-4, to expression on the myeloid and plasmacytoid DC subsets retrieved directly from blood with BDCA-1 (CD1c) and BDCA-4 (neuropilin)-positive selection (35). As reported, a high level of DC-SIGN was found on DCs derived from CD14+ monocytes, but it is known that DC-SIGN expression is induced by IL-4 (31), which is frequently used to differentiate monocytes into DCs. We confirmed that low doses of IL-4 alone rapidly induced DC-SIGN expression on CD14+ monocytes (Fig. 8A, left). DC-SIGN was not induced by GM-CSF, but GM-CSF and IL-4 together induced DC-SIGN at levels higher than IL-4 alone. Moreover, circulating blood myeloid DCs did not express DC-SIGN, but upon IL-4 treatment, DC-SIGN expression was again induced (Fig. 8A, right). In contrast, DC-SIGN was not expressed by the plasmacytoid DCs and was not induced after IL-4 treatment (not shown).
FIGURE 8.
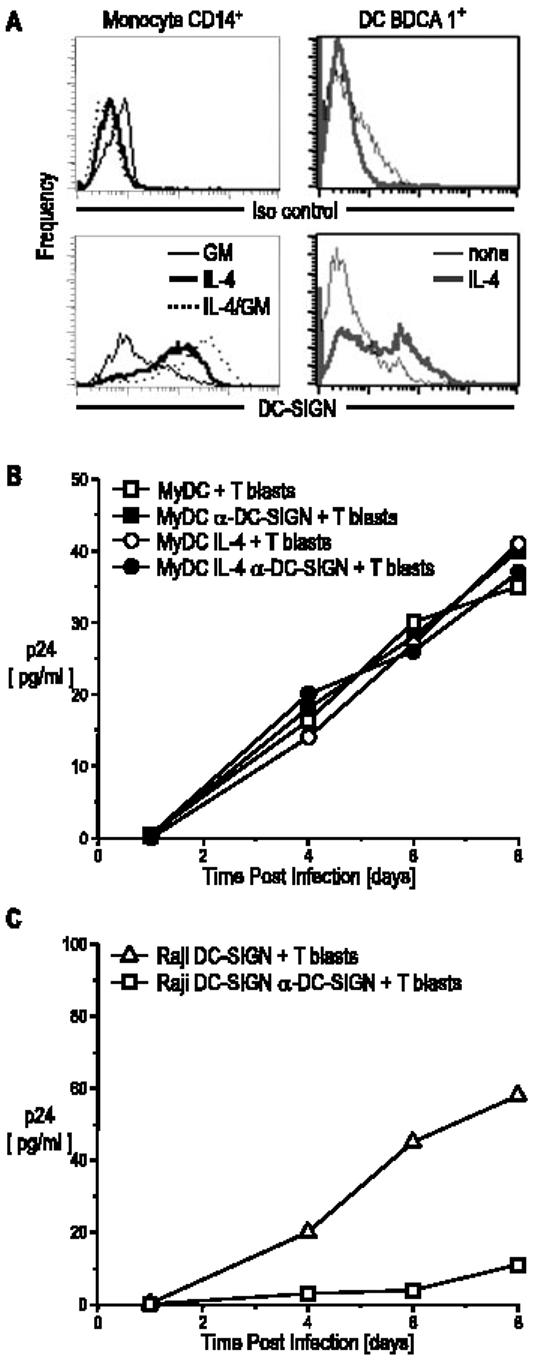
DC-SIGN expression is induced by IL-4 and is not required for HIV-1 transmission by blood myeloid DCs. A, CD14+ cells and BDCA1+ cells were cultured for 48 h with IL-4 (10 ng/ml), GM-CSF (100 IU/ml), or IL-4 and GM-CSF. Cells were stained with anti-DC-SIGN and analyzed by FACS. B, Myeloid DCs were cultured for 48 h with or without IL-4 (10 ng/ml), the latter to induce DC-SIGN expression. Part of the cultures were incubated for 20 min with 20 μg/ml anti-DC-SIGN mAb and AZN-D1 and then infected with BaL for 2 h and washed. Infected DCs (5 × 104) were cultured with 105 T blasts. Supernatants were collected at different time points and analyzed for p24 secretion by ELISA. C, Raji transfectants were studied in parallel.
To examine the involvement of DC-SIGN in HIV-1 capture and trans-enhancement by myeloid DCs from blood, we compared DCs that were or were not cultured in IL-4 to induce DC-SIGN as described above. After 48 h of IL-4 treatment to stimulate DC-SIGN expression, the myeloid DCs were incubated with or without anti-DC-SIGN mAb (AZN-D1), which has previously been shown to block infection via DC-SIGN in monocyte-derived DCs and Raji cells (4, 5). HIV-1 was then added for 2 h, the cells were washed extensively and cocultured with CD4+ T blasts, and the kinetics of viral replication were assessed by gag p24 release (Fig. 8B). Equally robust infection was observed in the presence or the absence of DC-SIGN induction or in the presence or the absence of anti-DC SIGN-blocking Ab, which did block DC-SIGN-transfected Raji cells studied in parallel (Fig. 8C). These results indicate that myeloid DCs from blood can transmit HIV to T cells independently from DC-SIGN.
DC-SIGN is not required for the response of T cells to DCs in the MLR
The allogeneic MLR is used as a model to study the capacity of DCs to initiate primary immune responses. Ab to DC-SIGN has been reported to block this response to some extent (1). We analyzed the effect of the panel of available anti-DC-SIGN mAbs during an MLR, monitored by the dilution of CFSE as an index of proliferation by CFSE-labeled CD3 T cells. The CFSE-labeled T cells were cultured with several ratios of allogeneic and syngeneic DCs that had been pretreated with anti-DC-SIGN mAbs, which were maintained throughout the culture at 20 μg/ml. The positive control for blocking of the MLR was an anti-HLA-DR Ab (clone L243), which profoundly inhibited T cell proliferation. However, none of our new anti-DC-SIGN mAbs had an inhibitory effect in all experiments similar to that shown in Fig. 9A. We then included an additional approach in parallel, which was to knock down DC-SIGN expression with a lentiviral vector expressing siRNA for DC-SIGN. Immature monocyte-derived DCs were infected at an MOI of 1:20 with siRNA DC-SIGN 11 (15), and after 48 h, the low DC-SIGN-expressing cells (Fig. 9B) were sorted to enrich the DC-SIGN-negative cells (Fig. 9C). These DC-SIGNlow DCs were not altered in their MLR-stimulating activity, and in addition, the other available sources of anti-DC-SIGN mAbs were unable to block MLR stimulation (Fig. 9D). We failed to observe inhibition over a range of DC to T cell ratios (1:10 to 1:100) and using both immature monocyte-derived DCs (obtained by monocyte culture with GM-CSF and IL-4) and mature DCs (obtained by treating the immature DCs with a mixture of IL-1β, IL-6, TNF-α, and PGE2). Taken together, our results do not show an involvement of DC-SIGN in DC-induced T cell activation in the MLR.
FIGURE 9.
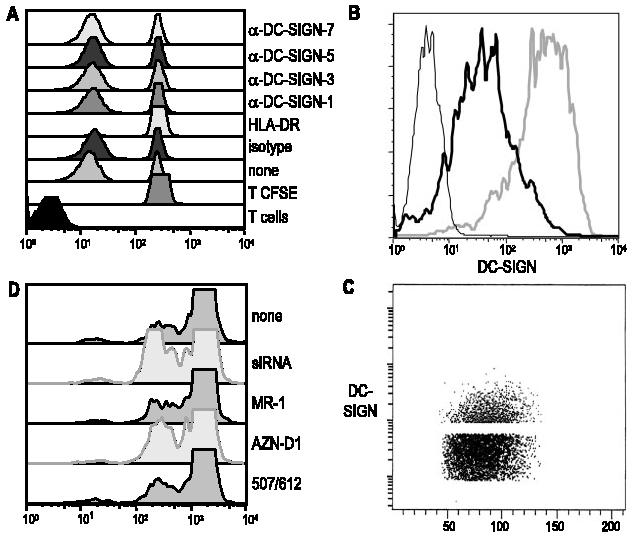
Inability of DC-SIGN to contribute to DC stimulation of the MLR. A, Immature DCs were pretreated for 20 min at room temperature with 20 μg/ml anti-DC-SIGN mAbs. DCs were cultured with allogeneic CFSE-labeled CD3 T cells at a ratio of 1:60. After 5 days, the presence of an MLR was documented by CFSE dilution. B, Immature DCs were transduced with siRNA DC-SIGN lentiviral vector or empty vector at an MOI of 20 and after 48 h were stained for DC-SIGN. The siRNA DC-SIGN (thick black line), empty vector (gray line), and isotype control (thin black line) are indicated. C, The low DC-SIGN-expressing cells were sorted and separated on a FACS. D, DC-SIGNlow sorted immature DCs were used to stimulate an MLR as described in A, and additional anti-DC-SIGN mAbs were tested for inhibition of the MLR.
Discussion
DC-SIGN/CD209 is a C-type lectin that is able to bind and transmit HIV-1, as discovered by Geijtenbeek et al. (4), and it has been implicated in the capacity of DCs to stimulate resting T cells (1). To assess the function of DC-SIGN in the noninflamed human lymph node, we have used a panel of anti-DC-SIGN mAbs, including new reagents obtained by immunization with recombinant vaccinia-DC-SIGN. Surprisingly, we found that DC-SIGN staining is primarily observed on macrophages throughout the medullary sinuses, as manifest by strong colabeling for two molecules that are abundant on macrophages, the CD68 lysosomal membrane protein and the mannose receptor, and a lack of DC markers such as CD205 and CD11c. In contrast, DCs in the T cell area of the cortex express high levels of CD205 and CD11c, but were not detectably stained with several new and previously available anti-CD209 mAb. Our approaches cannot determine whether DC-SIGN is not expressed at all, but only that the major depot of CD209 in the human lymph node are macrophages in the medulla and not DCs in the cortex. It will be important to learn how to isolate these cells from the lymphoid tissues, but at this time we have not succeeded in releasing significant numbers of DCs or macrophages with the very high levels of CD205 and CD209 that we observed in sections. Other reports have called attention to the detection of DC-SIGN on macrophages in lung and other sites (19, 36, 37), but we report that in the noninflamed lymph node, DC-SIGN is abundant on most macrophages in the medulla, but not in most DCs in the T cell areas.
Our findings in sections are surprising relative to the previous literature. Geijtenbeek et al. (1) identified some DC-SIGN+ cells in the T cell areas. Lore et al. (23) reported DC-SIGN+ cells in the parafollicular T cell-rich areas of lymph nodes from patients with HIV and EBV infection, and that these cells were reduced in healthy controls. Soilleux et al. (19) used a polyclonal anti-DC-SIGN serum to examine adult and fetal tissues and reported some cells with a dendritic morphology in the T cell areas and within sinuses in the cortex. Tailleux et al. (38) studied DC-SIGN expression in lymph nodes from tuberculosis patients and reported DC-SIGN+ cells within the granulomas and subcapsular sinuses. Engering et al. (24) reported DC-SIGN+ cells in the outer cortex in proximity to sinuses. While our study was under review, Krutzik et al. (37) described how macrophages in leprosy lesions could express DC-SIGN. Our paper, for the first time, reports the abundance of this lectin in medullary macrophages in noninflamed lymph nodes and its paucity on many T cell area DCs.
Our tissue section results indicate that many DCs in the T cell areas lack the markers of monocyte-derived DCs, particularly high expression of DC-SIGN/CD209 and mannose receptor/CD206. It is possible that the equivalent of the cultured monocyte-derived DCs only develops under select circumstances, e.g., during an inflammatory response or in special tissue niches. We and others (19, 31, 37, 39) found that cytokines such as IL-4, IL-13, and IL-15 quickly up-regulated DC-SIGN expression on monocytes, so that cells with the phenotype of monocyte-derived DCs might accumulate in situations such as parasite infection and allergy. The reports of DC-SIGN+ cells in lymphatic sinuses, particularly in the subcapsular region of lymph nodes, may also reflect migratory monocyte-derived DCs responding to a stimulus in the periphery. Within the T cell areas, we did detect small foci in which there were CD11c- and DEC-205-positive DCs that coexpressed DC-SIGN. However, most DCs in the T cell area had features shared with the myeloid subset of DCs in blood, i.e., the cells expressed CD11c and DEC-205/CD205, but lacked DC-SIGN and mannose receptor. We propose that the finding of DC-SIGN-positive DCs in the T cell areas represent situations where the equivalent of monocyte-derived DCs are being generated in vivo, but that the major reservoir of DC-SIGN in normal lymph node is the medullary sinus macrophage rather than cortical DCs.
With the anti-DC-SIGN Abs we have generated and those already characterized, we also restudied the role of DC-SIGN as a factor that participates in two types of DC-T cell interaction. All the anti-DC-SIGN mAbs tested, in contrast to anti-HLA-DR, were unable to inhibit DC-induced proliferation of resting T cells in the MLR. These results differ from the initial conclusion that DC-SIGN supports primary immune responses that arose from the observation that anti-DC-SIGN mAbs could reduce MLR stimulation by ∼60% (1). However, we were unable to detect a block of the MLR with a panel of anti-DC-SIGN mAbs even at limiting doses of DCs and duration of MLR. We also looked at the contribution of DC-SIGN to transmission of HIV by monocyte-derived DCs and by blood DCs that had been induced to express DC-SIGN with IL-4. We found that the mAbs to DC-SIGN did not impose a major reduction on transmission of HIV by these DCs, whereas the same mAbs led to a major ∼90% reduction of HIV transmission by DC-SIGN-transfected Raji cells. In both the MLR and HIV transmission assays, we additionally evaluated DCs in which DC-SIGN expression had been dampened with siRNA, and again, no blockade was noted, even though this approach markedly reduced HIV transmission from Raji DC-SIGN transfectants. These results are consistent with several other reports that molecules other than DC-SIGN can mediate virus transmission from DCs to T cells, using monocyte-derived DCs (6, 14, 16-18, 40, 41). Likewise, there are types of DCs that lack DC-SIGN, i.e., Langerhans cells and both myeloid and plasmacytoid DCs in blood, that are able to transmit HIV to T cells in vitro. Clearly, DC-SIGN represents an exciting new mechanism by which pathogens are recognized by innate cells, but DCs use additional mechanisms to transmit HIV, and we suggest that macrophages within lymph nodes be considered in pursuing the functions of DC-SIGN in vivo.
Acknowledgments
We are grateful to Yvette van Kooyk (Amsterdam, The Netherlands) and Angel Corbi (Madrid, Spain) for samples of anti-DC-SIGN Abs, to Dan Littman (New York University, New York, NY) for Raji-DC-SIGN transfectants, and Jane McKeating for L-SIGN transfectants. We thank Dolca Thomas for providing the lymph node specimens.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This work was supported by Direct Effect, Center for AIDS Research Grant 5P30AI42848-04 and National Institutes of Health Grants R01AI40045 and MO-1RR00102 (to Rockefeller University General Clinical Research Center).
Abbreviations used in this paper: DC-SIGN, dendritic cell-specific ICAM 3-grabbing nonintegrin; MDCK, Madin-Darby canine kidney; MOI, multiplicity of infection; siRNA, small interfering RNA.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Geijtenbeek TBH, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 2.Van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 3.Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA. 1992;89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell specific HIV-1 binding protein that enhances TRANS-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 5.Kwon DS, Gregario G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–144. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 6.Trumpfheller C, Park CG, Finke J, Steinman RM, Granelli-Piperno A. Cell type dependent retention and transmission of HIV-1 by DC-SIGN. Int. Immunol. 2003;15:289–298. doi: 10.1093/intimm/dxg030. [DOI] [PubMed] [Google Scholar]
- 7.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 8.Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B, Leslie G, Soilleux E, O'Doherty U, Baik S, Levroney E, Flummerfelt K, Swiggard W, Coleman N, Malim M, et al. Cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 2001;75:12028–12038. doi: 10.1128/JVI.75.24.12028-12038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 11.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman RM. Low levels of HIV-1 in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman D, Li Y, Orenstein JM, Fauci AS. Both a precursor and a mature population of dendritic cells can bind HIV: however, only the mature population that expressed CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 1995;155:4111–4117. [PubMed] [Google Scholar]
- 13.Granelli-Piperno A, Finkel V, Delgado E, Steinman RM. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 1999;9:21–29. doi: 10.1016/s0960-9822(99)80043-8. [DOI] [PubMed] [Google Scholar]
- 14.Baribaud F, Pohlmann S, Leslie G, Mortari F, Doms RW. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 2002;76:9135–9142. doi: 10.1128/JVI.76.18.9135-9142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrighi JF, Pion M, Wiznerowicz M, Geijtenbeek TB, Garcia E, Abraham S, Leuba F, Dutoit V, Ducrey-Rundquist O, van Kooyk Y, et al. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J. Virol. 2004;78:10848–10855. doi: 10.1128/JVI.78.20.10848-10855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gummuluru S, Rogel M, Stamatatos L, Emerman M. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 2003;77:12865–12874. doi: 10.1128/JVI.77.23.12865-12874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Bashirova AA, Martin TD, Villamide L, Mehlhop E, Chertov AO, Unutmaz D, Pope M, Carrington M, KewalRamani VN. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA. 2002;99:1568–1573. doi: 10.1073/pnas.032654399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 19.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage sub-populations in situ and in vitro. J. Leukocyte Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 20.Ebner S, Ehammer Z, Holzmann S, Schwingshackl P, Forstner M, Stoitzner P, Huemer GM, Fritsch P, Romani N. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int. Immunol. 2004;16:877–887. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- 21.Soilleux EJ, Morris LS, Lee B, Pohlmann S, Trowsdale J, Doms RW, Coleman N. Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV. J. Pathol. 2001;195:586–592. doi: 10.1002/path.1026. [DOI] [PubMed] [Google Scholar]
- 22.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lore K, Sonnerborg A, Brostrom C, Goh LE, Perrin L, McDade H, Stellbrink HJ, Gazzard B, Weber R, Napolitano LA, et al. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–692. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 24.Engering A, van Vliet SJ, Hebeda K, Jackson DG, Prevo R, Singh SK, Geijtenbeek TB, van Krieken H, van Kooyk Y. Dynamic populations of dendritic cell-specific ICAM-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am. J. Pathol. 2004;164:1587–1595. doi: 10.1016/S0002-9440(10)63717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector: 1982. Biotechnology. 1992;24:495–499. [PubMed] [Google Scholar]
- 28.Fernandez-Sesma A, Schulman JL, Moran TM. A bispecific antibody recognizing influenza A virus M2 protein redirects effector cells to inhibit virus replication in vitro. J. Virol. 1996;70:4800–4804. doi: 10.1128/jvi.70.7.4800-4804.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galustian C, Park CG, Chai W, Kiso M, Bruening SA, Kang YS, Steinman RM, Feizi T. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int. Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- 30.Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de La Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 32.Guo M, Gong S, Maric S, Misulovin Z, Pack M, Mahnke K, Nussenzweig M, Steinman RM. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum. Immunol. 2000;61:729–738. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 33.Bashirova AA, Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Eilering JB, Martin MP, Wu L, Martin TD, Viebig N, Knolle PA, et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes hiv-1 infection. J. Exp. Med. 2001;193:671–678. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soilleux EJ, Barten R, Trowsdale J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 2000;165:2937–2942. doi: 10.4049/jimmunol.165.6.2937. [DOI] [PubMed] [Google Scholar]
- 35.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 36.van Lent PL, Figdor CG, Barrera P, van Ginkel K, Sloetjes A, van den Berg WB, Torensma R. Expression of the dendritic cell-associated C-type lectin DC-SIGN by inflammatory matrix metalloproteinase-producing macrophages in rheumatoid arthritis synovium and interaction with intercellular adhesion molecule 3-positive T cells. Arthritis Rheum. 2003;48:360–369. doi: 10.1002/art.10786. [DOI] [PubMed] [Google Scholar]
- 37.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tailleux L, Schwartz O, Herrmann J-L, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman CJ, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chehimi J, Luo Q, Azzoni L, Shawver L, Ngoubilly N, June R, Jerandi G, Farabaugh M, Montaner LJ. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J. Leukocyte Biol. 2003;74:757–763. doi: 10.1189/jlb.0503231. [DOI] [PubMed] [Google Scholar]
- 40.Turville SG, Arthos J, Mac Donald K, Lynch G, Naif H, Clark G, Hart D, Cunningham AL. HIV gp120 receptors on human dendritic cells. Blood. 2001;98:2482–2488. doi: 10.1182/blood.v98.8.2482. [DOI] [PubMed] [Google Scholar]
- 41.Turville S, Wilkinson J, Cameron P, Dable J, Cunningham AL. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukocyte Biol. 2003;74:710–718. doi: 10.1189/jlb.0503208. [DOI] [PubMed] [Google Scholar]