Abstract
CD154 is a cell surface molecule expressed on activated T cells that binds to CD40, an activating molecule on APCs. Its blockade has been shown to prevent allograft rejection, presumably by interrupting interactions between T cells and APCs. It is known that activated human platelets express and shed CD154 and can induce APC activation and other immune processes in vitro. Here we show that platelet-derived CD154 is sufficient to initiate cardiac allograft rejection independent of any cellular source of this molecule. CD154-KO mice reject cardiac allografts after receiving CD154-expressing human platelets or recombinant CD154 (rCD154) trimers. Treatment with the human CD154-specific mAb 5c8 specifically prevents this induced rejection. Soluble trimers, but not platelets, induce rejection when infused temporally remote from the surgical procedure, suggesting that surgically induced platelet activation is required for CD154 release. Allograft rejection can thus be instigated by activated platelets through CD154. These data implicate platelets as a proximal component of acquired alloimmunity, providing insight into the mechanisms of allograft rejection and the physiological response to trauma in general.
Introduction
CD154 is a 33-kDa type II transmembrane protein originally identified on activated T cells as the ligand for CD40, a molecule promoting B cell and APC activation (1). CD154 has been shown to play a critical role in the generation of alloimmune responses. Specifically, treatment with CD154-specific mAbs prevents acute allograft rejection in both murine and nonhuman primate models, presumably by blocking T cell interactions with APCs expressing CD40 (2–7). However, CD154-directed therapy has also been shown to require high levels of antibody to achieve an effect, greatly exceeding those typically required of antibodies targeting T cell activation markers (8). Furthermore, unlike antibody therapy directed toward T cell molecules, treatment with CD154-specific antibodies has been associated with thromboembolic complications (9).
It has been recently shown that human platelets contain preformed CD154 and express and shed this molecule upon activation (10). Both cell-surface–bound and soluble forms of CD154 exist as homotrimers (11, 12), and soluble platelet-derived CD154 has been shown to mediate endothelial and APC activation in vitro (10, 13–15). Indeed, CD154 cleaved and released in soluble form after platelet activation has been considered a major source of circulating CD154 (16). Furthermore, soluble CD154 has increasingly been associated with adverse inflammatory conditions in humans, including lupus and acute coronary syndrome (17, 18). To date, however, there has been no direct evidence in vivo that soluble CD154 can mediate pathological immune responses independent of cell-bound CD154.
Organ transplantation is, by necessity, associated with surgical trauma. Given that biologically active CD154 is present in platelets and released upon activation and that platelet activation is unavoidable in any surgical procedure, we have hypothesized that platelet activation induced during organ transplantation contributes significantly to allograft rejection through the CD154 pathway (14, 19, 20) and that soluble CD154 significantly influences the efficacy of CD154-targeted therapies. We have thus explored the role of non–T cell–derived CD154 in vascularized allograft rejection to determine if it induces rejection in vivo. We found that human platelet–derived or soluble recombinant CD154 (rCD154) induces cardiac allograft rejection independent of any cell-bound source of this molecule and that platelets can initiate alloimmunity through CD154 release when activated at the time of a surgical procedure.
Results
Human CD154 is biologically active in a murine environment.
We first determined whether human CD154 had biological activity in mice such that cell-bound and soluble forms of the human molecule could be clearly distinguished and independently studied in vivo. We evaluated 1-way mixed lymphocyte reactions (MLRs) using WT C57BL/6 or CD154-KO (B6.129S2-Tnfsf5) splenocytes as responders and fully MHC-mismatched BALB/c splenocytes as stimulators in the presence or absence of human rCD154 trimers. As expected, CD154-KO splenocytes had attenuated proliferative responses to BALB/c splenocytes compared with WT splenocytes, and CD154 blockade with a murine CD154–specific mAb, MR-1, inhibited proliferation of WT splenocytes (data not shown). The addition of human rCD154 trimers induced dose-dependent proliferation of CD154-KO splenocytes but only when concomitantly stimulated by BALB/c splenocytes (data not shown). This proliferative response was not inhibited by MR-1, which is only specific for murine CD154, but was inhibited by the human-specific mAb 5c8 (Figure 1). Isotype control antibodies for both MR-1 and 5c8 did not block lymphocyte proliferation, and the human rCD154 trimers, when incubated with CD154-KO murine splenocytes without allogeneic antigens, induced minimal lymphocyte proliferation (data not shown). CD154-KO spleens were evaluated by flow cytometry and shown to have ample numbers of CD4- and CD8-positive T cells. Thus, the attenuated response of CD154-KO splenocytes was not attributable to relative T cell deficiency in these mice.
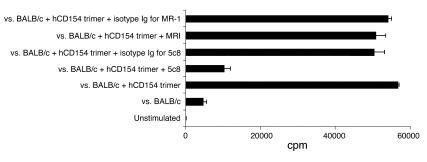
Figure 1.
Human CD154 (hCD154) induces cellular proliferation in mice that is blocked by human CD154–specific but not murine CD154–specific antibodies. Shown are results from MLR using B6-CD154-KO responders (B6.129S2-Tnfsf5) versus BALB/c stimulators with or without human rCD154 trimers (50 μg/ml) and/or the human- or murine-specific CD154 mAbs. KO lymphocytes respond poorly in MLRs, and this response is greatly augmented by the addition of human CD154 trimers. This proliferation is specifically blocked by the human-specific antibody 5c8 but not by the murine-specific antibody MR-1 or isotype controls.
To further define the extent to which human CD154 could influence murine responses, we evaluated human CD154 trimer interactions with murine DCs. We stimulated murine DCs, isolated from B6.129S7-Rag1 mice, with human rCD154 trimers in the presence or absence of recombinant murine IFN-γ for 24 hours. The supernatants were collected and analyzed by ELISA for murine IL-12 (p70), an indicator of DC activation. LPS was used as a positive control. Human CD154 trimers induced production of IL-12, an effect that was further augmented by the presence of murine IFN-γ (data not shown). These results indicate that soluble human CD154 trimers sufficiently cross-react with murine CD40 to initiate allogeneic cell–mediated immune responses. Thus, human soluble CD154 could be investigated independent of any cell-bound sources of CD154 in a murine system.
Since it has been shown that human CD154 can, in addition to activating APCs, also activate human ECs, we also sought to determine if human CD154 activated murine endothelium. Cultured murine EC monolayers were stimulated with human CD154 trimers at 100 μg/ml for 2, 4, and 24 hours followed by FACS analysis for CD54 and CD62E. Recombinant murine TNF-α served as a positive control. Human CD154 trimers stimulation did not lead to upregulation of CD54 beyond its basal expression (data not shown) and did not lead to CD62E expression (Figure 2). Thus, human CD154 activates murine APCs but not murine ECs, and its effects are thus indicative of immune activation rather than changes in EC adhesion.
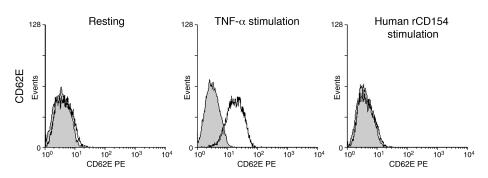
Figure 2.
Soluble rCD154 trimers (100 μg/ml) do not activate murine vascular ECs. Shown are flow cytometry analyses of murine ECs stained for CD62E following stimulation with TNF-α (a positive control) or human rCD154.
Soluble CD154 initiates vascularized allograft rejection.
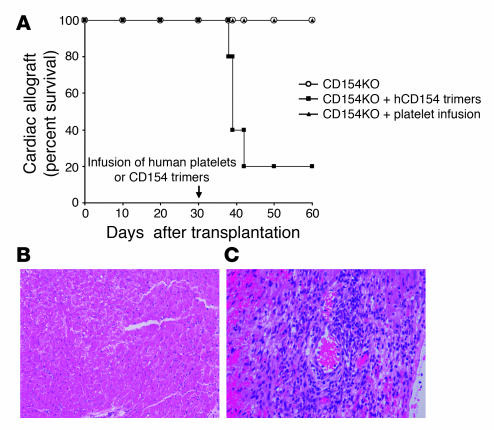
To evaluate the ability of soluble CD154 to initiate allograft rejection, the effects of human rCD154 trimers were tested in vivo in a well-established murine heterotopic cardiac transplant model (21). C57BL/6 recipients (n = 10) rejected BALB/c cardiac allografts in a mean of 8.2 days (Figure 3A). Treatment with a single 1 mg dose of MR-1 prevented acute cardiac allograft rejection for more than 80 days (n = 10; Figure 3A). CD154-KO recipients similarly experienced long-term cardiac allograft survival with 80% of the animals surviving for more than 100 days (n = 14; Figure 3A). In contrast, when CD154-KO animals received a single intravenous infusion of human rCD154 trimers (500 μg) at the time of transplantation, 11 of 12 rejected their allografts on postoperative days 8 to 34 (P < 0.001 versus untreated KO animals; Figure 3A). Additionally, this trimer-induced rejection was prevented in 5 of 5 CD154-KO recipients by treatment with the human-CD154–specific antibody 5c8 (200 μg on days –1, 0, 3, 5, and 7), initiated before trimer infusion (P = 0.002 versus trimer-treated KO animals; Figure 3A). Trimer injection into WT animals significantly decreased the time to rejection from a mean of 8.2 days to a mean of 6.8 days (n = 6; P = 0.001; Figure 3A).
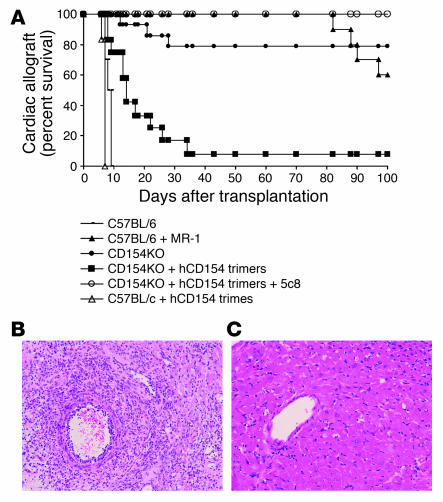
Figure 3.
Soluble rCD154 trimers induce cardiac allograft rejection in CD154-deficient mice. (A) C57BL/6 recipients accept BALB/c donor heart allografts with (n = 10) but not without (n = 10) CD154 blockade using MR-1. Similarly, CD154-KO mice accept cardiac allografts (n = 14). However, the infusion of human rCD154 trimers (500 μg) induces rejection by CD154-KO recipients (n = 12; P < 0.001 versus untreated animals). Human CD154 blockade with the anti-human CD154 antibody 5c8 restores the tolerant phenotype to animals receiving CD154 trimers (n = 5; P = 0.002 versus trimer-treated animals). Infusion of trimers into WT animals (n = 6) accelerated the rate of rejection by an average of 1.4 days (P = 0.001 versus untreated controls). (B) Cardiac allografts from CD154-KO recipients that received human rCD154 trimers show acute cellular rejection. Magnification, ×20. (C) Allografts from CD154-KO recipients treated with 5c8 followed by infusion of human rCD154 trimers show normal myocardium without rejection. Magnification, ×40.
Histological evaluation of the rejected cardiac allografts showed acute cellular rejection with a typical lymphocyte infiltration and vascular endothelial cell activation both in WT untreated animals (data not shown) and in CD154-KO recipients that received rCD154 trimers (Figure 3B). In contrast, cardiac allografts harvested from CD154-KO recipients treated with CD154 trimers and 5c8 showed no evidence of acute rejection even 100 days after transplantation (Figure 3C). These observations demonstrate that a soluble form of human CD154 can initiate cardiac allograft rejection without any cell-bound source of this molecule.
Platelets can serve as the sole source of CD154 in allograft rejection.
To directly test whether CD154 released in response to surgical trauma initiates allograft rejection, we obtained purified resting human platelets and verified the absence of CD154 in a resting state and their ability to express CD154 following stimulation by human thrombin or thrombin receptor activator peptide (TRAP-1) (data not shown). To evaluate the fate of human platelets in mice, human platelets were labeled with PKH-26 and then adoptively transferred into CD154-KO mice via tail vein injection. Flow cytometry demonstrated that platelets were cleared from the circulation within 24 hours (data not shown). Histological evaluation of spleens collected from these mice revealed PKH-26–positive platelets located predominantly in the splenic red pulp within the first 24 hours (Figure 4A) that persisted for at least 14 days (data not shown). Platelets were also detected in the interstitium of the allograft 1 day after transplantation (Figure 4A) and to a lesser degree in the periaortic lymph nodes (data not shown).
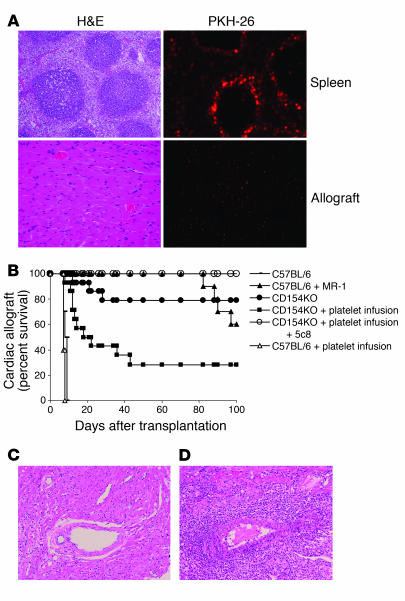
Figure 4.
CD154-deficient mice reject cardiac allografts following human platelet transfusion. This is blocked by human CD154–specific 5c8. (A) PKH-26–labeled human platelets aggregate in the splenic red pulp and are also seen in the interstitium of the transplanted heart 24 hours following intravenous infusion. Shown is an H&E-stained section and its corresponding fluorescence image for spleen and cardiac allograft tissue derived from a mouse following injection of PKH-26–stained platelets. (B) CD154-KO recipients (n = 28) received human platelet transfusions followed by cardiac allografts; half were treated with 5c8. Animals that received platelets rejected (P = 0.008 versus nontransfused animals), but those pretreated with 5c8 did not (P = 0.012 versus untreated platelet-transfused animals). Infusion of platelets into WT animals (n = 5) accelerated the rate of rejection by 0.8 days (P = 0.057). Control groups C57BL/6, C57BL/6 + MR-1, and CD154KO represent the same animals described in the Figure 3 legend and are displayed in this graph for comparative purposes. (C) Cardiac allograft rejection in CD154-KO recipients that received human platelet transfusions. Magnification, ×20. (D) Allografts harvested from CD154-KO recipients treated with anti-CD154 5c8 followed by infusion of human platelets are protected from rejection and are histologically similar to cardiac allografts collected from untreated CD154-KO recipients that do not reject (not shown). Magnification, ×20.
We then adoptively transferred resting human platelets into CD154-KO recipients (n = 28) followed by cardiac allografts from BALB/c donors. Half of these recipients were also treated with the human CD154-specific mAb 5c8 (200 μg on days –1, 0, 3, 5, and 7). Ten of 14 CD154-KO recipients that received human platelets promptly rejected their cardiac allografts (P = 0.008 versus untreated KO animals; Figure 4B), with histological findings that were indistinguishable from control rejecting animals (Figure 4C). Treatment with 5c8 prevented cardiac allograft rejection in all animals (P = 0.012 versus platelet-treated KO animals; Figure 4B). At sacrifice, these animals had no histological evidence of acute rejection (Figure 4C), and their histology was similar to that seen in CD154-KO recipients that did not receive trimers or platelet infusions. As with trimer infusion, infusion of human platelets into WT recipients modestly accelerated the pace of acute rejection to a mean of 7.4 days (n = 5; P = 0.057; Figure 4B).
To determine the role of surgical trauma in activating platelets, CD154-KO animals (n = 14) were given cardiac allografts. The animals were allowed to heal for 30 days, at which time platelets were adoptively transferred (n = 5) or CD154 trimers were infused (n = 5). Mice (n = 4) without platelet or trimer infusion were also studied 1 month after transplant for baseline histological evaluation. Unlike those animals that received platelet transfusions at the time of surgery, animals receiving platelet infusions 30 days after transplantation maintained stable allograft function (Figure 5A). These animals were sacrificed at days 30 and 60 after platelet infusion, and histological evaluation showed no evidence of rejection (data not shown). In contrast, 4 of 5 mice receiving CD154 trimer infusions 1 month after cardiac transplantation rejected their allografts within 8 to 12 days (P = 0.008 versus untreated animals; Figure 5, A and B). The control mice without human platelet or CD154 trimer infusion were euthanized at posttransplant day 30 and did not have rejection (Figure 5C). Thus, soluble CD154 can induce rejection late after transplantation, but platelets are unable to provide sufficient CD154 in the absence of a concomitant activating event such as surgery or reperfusion.
Figure 5.
When infused 30 days after surgery, soluble rCD154 induces rejection but platelets do not. (A) CD154KO animals that received rCD154 trimers 30 days after transplantation promptly rejected their allografts (n = 5; P = 0.008 versus untreated animals). Animals receiving platelet transfusions 30 days after transplantation did not reject (n = 5). (B) CD154-KO recipients that received human platelet transfusions on day 30 were euthanized 30 days after infusion and showed no evidence of rejection. Magnification, ×20. (C) Those receiving trimers had histologically typical rejection. Magnification, ×40.
Discussion
Our data demonstrate for the first time, to our knowledge, that human soluble CD154 is capable of inducing a pathological immune response in vivo, specifically allograft rejection, independent of any cell-bound source of this critical costimulatory molecule. Furthermore, we have shown that human platelets activated in vivo at the time of transplantation induce rejection through CD154. Given the inseparable relationship between surgical trauma and organ transplantation, these findings highlight a novel and potentially important mechanism for alloimmune response initiation and suggest a more general relationship between CD154 and other detrimental posttraumatic immune responses. These findings are relevant to the transition of CD154-based therapies into clinical practice and suggest new therapeutic avenues for their application.
CD40 has been firmly established as a critical molecule mediating professional (e.g., monocyte, DC) and opportunistic (e.g., EC) APC function (22–26). The ability of platelet-derived CD154 to mediate these events as well as effector antibody and T cell maturation has become more recently apparent (10, 12–14). The current study underscores the importance of this pathway in a clinically relevant pathological immune response, organ rejection, and highlights the considerable potential for platelets to participate in other posttraumatic immune responses, both protective and detrimental. Although the controlled circumstance of an elective surgical procedure is not typically viewed as traumatic, CD154 provides a mechanism for immune activation to be stirred proportional to the injury sustained. Indeed, platelet/monocyte complexes have recently been shown to form following elective surgical procedures (27), and there is a known association between worsening ischemic insult and increased rejection rate (28). The accelerated rejection seen when trimers or platelets were added to WT grafts in this study demonstrates that soluble CD154 can be immunostimulatory even in the WT setting and augment immunity in a manner proportionate to its release. Thus, while it is clear that immune responses such as allograft rejection are not solely predicated on trauma (29), the concept of injury as a driving force behind augmented immunity is easily reconciled with the present findings.
The findings from these studies do not negate the importance of cell-bound CD154 in mediating immune responses. Clearly, CD154 expression on activated T cells influences immune responsiveness through its effects on APCs and also perhaps through direct effects on the T cell itself (30, 31). However, they do add considerably to the magnitude of immune induction possible under certain circumstances. Although CD154 is an inducible molecule on the cell surface, the magnitude of its induction through T cell activation is fixed by the potential number of responding antigen-specific T cells and the rate and degree to which these cells are activated after antigen presentation. Soluble CD154 derived from platelets potentially provides a considerable amount of this ligand independent of and prior to antigen presentation and cell activation. Thus, acquired immunity can be initiated and driven in response to clinical situations such as vascular injury or other traumatic conditions.
It remains unclear if there is a dominant anatomical site in which soluble CD154 mediates its effects. Clearly, the spleen showed the highest concentration of infused platelets in our model, although platelets were also detected in the allograft and to a lesser degree in periaortic lymph nodes. The distribution of trimers is presumably similar to other large macromolecules and is likely broader. The spleen is a known site for alloimmune activation following primarily vascularized transplantation (32), and the effects seen in our study could be explained by augmented antigen presentation in the spleen. However, platelets are intravascularly ubiquitous, and cogent arguments can be made for local CD154 in the graft-stimulating passenger donor APCs and migratory monocytes. Since primarily vascularized allografts have disrupted lymphatic drainage, it is difficult to determine the role of a true draining lymph-node bed for most organ grafts, although activated platelets could have a role in nodal tissue as well. We favor a primary role for the spleen during systemic activation as would be expected following a major surgical procedure and are investigating the local and systemic effects of activated platelets.
Based on these data, it is evident that for CD154 blockade to effectively prevent an immune response in a traumatic condition, it must address a potentially large reservoir of platelet-derived CD154. Indeed, in dose-range studies, exceptionally large doses of mAb have been necessary to prevent rejection in nonhuman primates (8). Given the substantial concern regarding thromboembolic complications arising from the initial clinical trials with anti-CD154 (9, 13, 33), the effects of CD154 cross-linking on platelets will need to be cautiously examined. It may be more feasible to target CD40, a molecule with more limited distribution. Regardless, this non–T cell component must be considered in the design of antirejection therapies and should be scrutinized in other posttraumatic immune responses.
Methods
Mice.
We purchased 6- to 8-week-old male B6.129S2-Tnfsf5 and B6.129S7-Rag1 mice from Jackson Laboratory. Eight-week-old BALB/c and C57BL/6 mice were obtained from the National Cancer Institute. The experiments described in this study were conducted according to the principles set forth in the Guide for the care and use of laboratory animals (Institute of Laboratory Animals Resources, National Research Council, Department of Health and Human Services, NIH Pub. No. 86-23, 19850). All procedures were performed according to animal care and use protocols approved by the Intramural Institutional Animal Care and Use Committee of the National Institute of Diabetes, Digestive and Kidney Disease.
Cellular assays.
Splenocytes were isolated and MLRs were performed using 2 × 105 responders and mitomycin C–treated stimulators at 3 different ratios to responders or 2 × 105 stimulators. MLRs were carried out for 5 days and pulsed with 3H-thymidine at 1 μCi/well during the final 24-hour culture. The lymphocyte proliferation was determined as cpm by 3H-thymidine incorporation, using a β-liquid scintillation counter. The anti-human CD154 mAb 5c8 was used at 100 μg/ml for human CD154 blockade. The anti-murine CD154 mAb MR-1 was used at 100 μg/ml for murine CD154 blockade. Isotype control antibodies were hamster IgG (CALTAG Laboratories) and mouse IgG1 (Ancell Corp.).
To evaluate DC activation, murine bone marrow was harvested from the B6.129S7-Rag1 mouse (femur, tibia, and humerus) by flushing the marrow with RPMI-1640 medium, using a 6-ml syringe and 26G needle. Cells were washed after lysing red blood cells and incubated with culture medium containing 10% FCS, 10 ng/ml murine granulocyte/macrophage colony stimulating factor, and 10 ng/ml IL-4 (PeproTech) for 4 days. Nonadherent cells were collected and verified as DCs by positive staining for CD83, CD86, and HLA-DR (> 90%). DCs (5 × 104) were added into 96-well flat-bottom plates in the presence or absence of human rCD154 trimers (50 μg/ml; R&D Systems) or in combination with recombinant murine IFN-γ (2 ng/ml; PeproTech). Supernatants were collected after 24-hour stimulation and analyzed by ELISA to detect IL-12 production. DCs stimulated by LPS (60 ng/ml; Sigma-Aldrich) or without stimulation were used as positive and negative controls, respectively. The mouse IL-12 (p70) ELISA kit was obtained from BD Biosciences. An ELISA was performed according to the manufacturer’s instructions. The absorbance of each well was measured at 450 nm within 30 minutes of stopping reaction.
To evaluate direct effects of human CD154 trimers on murine ECs, the BALB/c bEnd.3 EC cell line was obtained from ATCC and maintained with Dulbecco’s modified Eagle’s medium (Invitrogen Corp.) supplemented with 10% FCS. For positive control activation, ECs were treated with TNF-α, and activation was verified by upregulation of CD54 and CD62E as determined by FACS using a FACScan (BD). Murine EC monolayers were stimulated with human CD154 trimers at 100 μg/ml at different time points followed by staining with mAbs directed to either CD62E or CD54 on ice for 30 minutes. Cells were washed twice with FACS buffer followed by incubation with PBS (Invitrogen Corp.) containing 20 mM HEPES (BioWhittaker Inc.), pH 7.4, 10 mM EDTA, and 0.5% BSA (Sigma-Aldrich) at 4°C for 20 minutes and then at 37°C for 20 minutes. Detached ECs were analyzed by FACScan.
Platelet preparation and stimulation.
Volunteers were enrolled in an NIH Institutional Review Board–approved tissue acquisition protocol and donated after informed consent was obtained. Purified human platelets were obtained from leukapheresis products from normal volunteers from the Department of Transfusion Medicine, NIH. Platelets were washed twice with Ca2/Mg2-free PBS containing 100 nM PGE-1 (Sigma-Aldrich) and 1.9 mM theophylline by centrifugation. Cells were diluted with PBS to 500 × 106/ml and incubated with 100 U/ml thrombin receptor activator peptide (TRAP) at room temperature for 10 minutes. Both resting and activated platelets were then stained with mouse IgG1-PE, anti-CD41a-PE, anti-CD62P-PE, and anti-CD154-PE mAbs at 4°C for 30 minutes followed by flow cytometric analysis.
To detect the presence of human platelets in CD154-KO mice following adoptive transfer, human platelets were labeled with PKH-26, a fluorescent compound that incorporates aliphatic reporter molecules into the cell membrane by selective partitioning, according to the protocol suggested by the manufacturer (Sigma-Aldrich). PKH-26–labeled platelets were evaluated by flow cytometric analysis before transfusion, and peripheral blood was evaluated up to 24 hours after infusion. Spleens, periaortic lymph nodes, and allografts were evaluated histologically 1 day after injection using H&E stains for general architecture and fluorescence microscopy to detect PKH-26–stained platelets. The spleens from nontransplanted animals were also evaluated at 4, 8, and 14 days after injection.
Cardiac transplants and treatments.
Transplants were performed heterotopically with vascularized BALB/c cardiac allografts as described using standard microvascular techniques (21). Cardiac allografts were monitored by daily palpation and removed for histological evaluation after cessation of allograft contractility. Human CD154 trimers were injected intravenously via tail vein before cardiac allografts. On days –1, 0, 3, 5, and 7, 200 μg 5c8 or MR-1 was injected i.p. Graft survival times were compared between groups using 2-tailed unpaired Student’s t tests with P < 0.05 considered as statistically significant.
Acknowledgments
The authors gratefully acknowledge Frank Leopardi for his expert technical assistance, Mark St. Clair for his veterinary care, and Ashish Jain for his critical reading of this manuscript. This work was funded by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH.
Footnotes
Nonstandard abbreviations used: MLR, mixed lymphocyte reaction; rCD154, recombinant CD154.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Armitage RJ, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 2.Parker DC, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 4.Hancock WW, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk AD, et al. CTLA-4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk AD, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat. Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, et al. Studies investigating pretransplant donor-specific blood transfusion, rapamycin, and the CD154-specific antibody IDEC-131 in nonhuman primate model of skin allotransplantation. J. Immunol. 2003;170:2776–2782. doi: 10.4049/jimmunol.170.5.2776. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, et al. Effects of dose and duration of anti-CD154 antibody therapy in preventing renal allograft rejection in a nonhuman primate model. Transplant. Proc. 2001;33:223–224. doi: 10.1016/s0041-1345(00)01983-7. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand [letter] Nat. Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 10.Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 11.Pietravalle F, et al. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J. Biol. Chem. 1996;271:5965–5967. doi: 10.1074/jbc.271.11.5965. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YM, et al. Heteromultimeric complexes of CD40 ligand are present on the cell surface of human T lymphocytes. J. Biol. Chem. 1997;272:911–915. doi: 10.1074/jbc.272.2.911. [DOI] [PubMed] [Google Scholar]
- 13.Elzey BD, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.Czpiaga M, Kirk AD, Lekstrom-Himes J. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp. Hematol. 2004;32:135–139. doi: 10.1016/j.exphem.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, et al. Human platelets activate porcine endothelial cells through a CD154-dependent pathway. Transplantation. 2001;72:1858–1861. doi: 10.1097/00007890-200112150-00029. [DOI] [PubMed] [Google Scholar]
- 16.Andre P, et al. CD40L stabilizes arterial thrombi by a β3 integrin-dependent mechanism. Nat. Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 17.Kato K, et al. The soluble CD40 ligand sCD154 in systemic lupus erythematosus. J. Clin. Invest. 1999;104:947–955. doi: 10.1172/JCI7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heeschen C, et al. CD40 ligand in acute coronary syndromes. N. Engl. J. Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 19.Kirk AD. Let’s blame the little guys: platelets as an instigator of allograft rejection? Graft. 1999;2:159–160. [Google Scholar]
- 20.Kirk AD, Blair PJ, Tadaki DK, Xu H, Harlan DM. The role of CD154 in organ transplant rejection and acceptance. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356:691–702. doi: 10.1098/rstb.2001.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corry RJ, Winn HJ, Russell PS. Primary vascularized allograft of hearts in mice. The role of H-2D, H2-K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Alderson MR, et al. CD40 expression by human monocytes: regulation by cytokine and activation of monocytes by the ligand for CD40. J. Exp. Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melter M, et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96:3801–3808. [PubMed] [Google Scholar]
- 24.Caux C, et al. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dechanet J, et al. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J. Immunol. 1997;159:5640–5647. [PubMed] [Google Scholar]
- 26.Karmann K, Hughes CCW, Schechner J, Fanslow WC, Pober AS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4342–4346. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunescu A, et al. Increases in circulating levels of monocyte-platelet and neutrophil-platelet complexes following hip arthroplasty. Clin. Sci. (Lond.). 2002;102:279–286. [PubMed] [Google Scholar]
- 28.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N. Engl. J. Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 29.Bingaman AW, et al. Vigorous allograft rejection in the absence of danger. J. Immunol. 2000;164:3065–3071. doi: 10.4049/jimmunol.164.6.3065. [DOI] [PubMed] [Google Scholar]
- 30.Hancock WW, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blair PJ, et al. CD40 Ligand (CD154) triggers a short-term CD4(+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J. Exp. Med. 2000;191:651–660. doi: 10.1084/jem.191.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J. Exp. Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boumpas DT, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]