Abstract
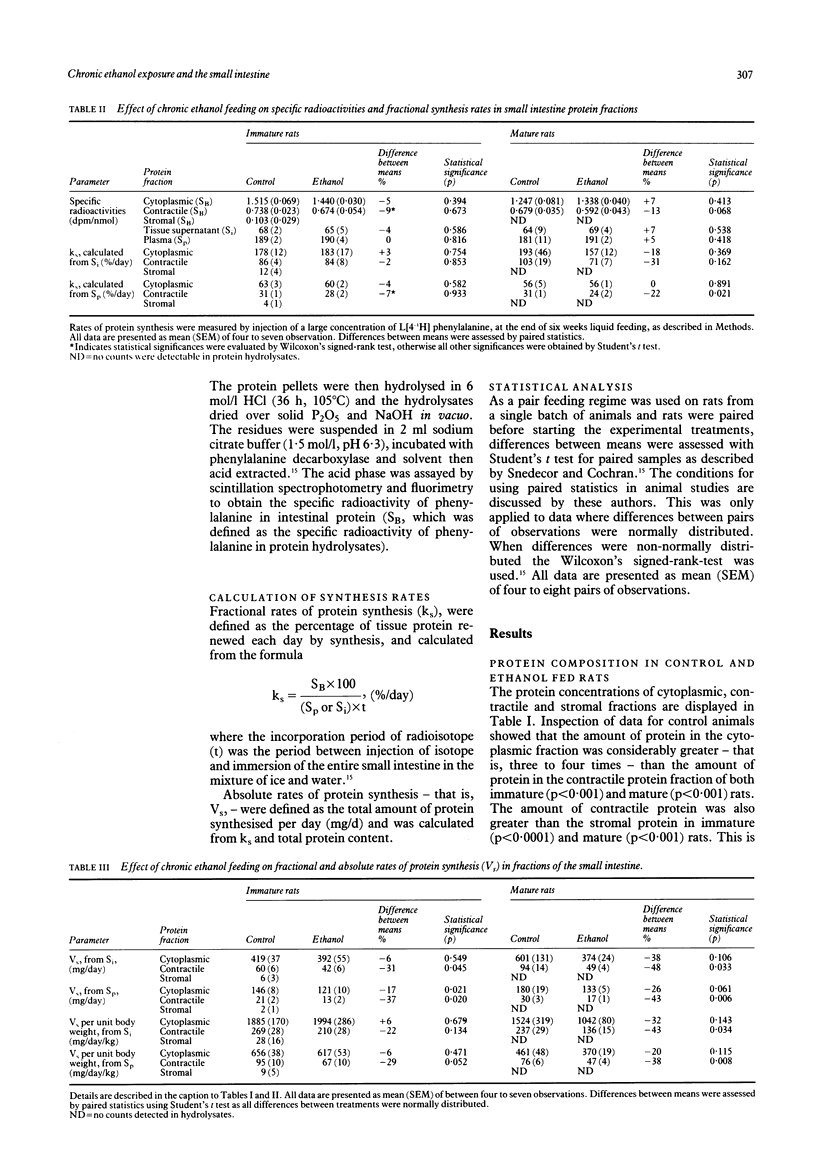
The effects of chronic ethanol feeding on the amounts and synthesis rates of cytoplasmic, contractile, and stromal protein fractions were investigated in the small intestine of eight pairs of immature and seven pairs of mature rats. Treated rats were fed ethanol as 36% of total energy in a nutritionally adequate liquid diet. Paired controls were fed isovolumetric amounts of the same diet in which ethanol was substituted by isocaloric glucose. After six weeks the total cytoplasmic and contractile protein content in immature rats was reduced by 18% and 31%, respectively (p less than or equal to 0.007). The decline in the stromal protein content (26%) was not statistically significant (p = 0.130). In mature rats the protein contents were also reduced in the cytoplasmic (25%, p = 0.035) and contractile (27%, p = 0.005) protein fractions, though the stromal protein fraction was unaltered (p = 0.913). In immature rats fractional rates of protein synthesis in cytoplasmic and contractile protein fractions of the small intestine were unaltered by chronic ethanol feeding (p less than or equal to 0.853). In mature rats, the synthesis rates of corresponding fractions declined, by 18% and 31%, respectively, but were also not statistically significant (p less than or equal to 0.369). Absolute rates of protein synthesis in immature rats fell by 6% (p = 0.549) in the cytoplasmic and 31% in the contractile protein fraction (p = 0.045). In mature rats, the corresponding reductions were 38% (p = 0.106) and 48% (p = 0.033), respectively. Virtually no radioactivity could be detected in the stromal fraction, signifying very low synthesis rates. Chronic ethanol feeding reduces the amount of protein in the small intestine of the immature and mature rat with the contractile protein fraction showing the greatest decrease. In the absence of statistically significant reductions in fractional synthesis rates a partial adaptation in turnover rates may have occurred.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel F., Sava P., Crenner F., Lambert A., Grenier J. F. Modifications de la motricité intestinale aprés injection intra-veineuse d'alcool. C R Seances Soc Biol Fil. 1980;174(2):192–198. [PubMed] [Google Scholar]
- Barona E., Pirola R. C., Leiber C. S. Small intestinal damage and changes in cell population produced by ethanol ingestion in the rat. Gastroenterology. 1974 Feb;66(2):226–234. [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Levi A. J., Peters T. J. Intestinal permeability to 51Cr-EDTA in rats with experimentally induced enteropathy. Gut. 1985 Jun;26(6):579–585. doi: 10.1136/gut.26.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A. J., Chalmers D. M. Recognition of alcoholic liver disease in a district general hospital. Gut. 1978 Jun;19(6):521–525. doi: 10.1136/gut.19.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell B. B., Ruderman N. B., Goodman M. N. Evidence that lysosomes are not involved in the degradation of myofibrillar proteins in rat skeletal muscle. Biochem J. 1986 Feb 15;234(1):237–240. doi: 10.1042/bj2340237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti R., Jenkins W. J. Effect of chronic ethanol ingestion on enterocyte turnover in rat small intestine. Gut. 1987 Jan;28(1):52–55. doi: 10.1136/gut.28.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Duane P., Peters T. J. Acute ethanol dosage reduces the synthesis of smooth muscle contractile proteins in the small intestine of the rat. Gut. 1988 Sep;29(9):1244–1248. doi: 10.1136/gut.29.9.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Duane P., Peters T. J. Biological effects of chronic ethanol consumption: a reappraisal of the Lieber-De Carli liquid-diet model with reference to skeletal muscle. Alcohol Alcohol. 1988;23(2):151–154. [PubMed] [Google Scholar]
- Preedy V. R., Paska L., Sugden P. H., Schofield P. S., Sugden M. C. The effects of surgical stress and short-term fasting on protein synthesis in vivo in diverse tissues of the mature rat. Biochem J. 1988 Feb 15;250(1):179–188. doi: 10.1042/bj2500179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy V. R., Peters T. J. Acute effects of ethanol on protein synthesis in different muscles and muscle protein fractions of the rat. Clin Sci (Lond) 1988 May;74(5):461–466. doi: 10.1042/cs0740461. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Peters T. J. The effect of chronic ethanol feeding on body and plasma composition and rates of skeletal muscle protein turnover in the rat. Alcohol Alcohol. 1988;23(3):217–224. [PubMed] [Google Scholar]
- Reeds P. J., Haggarty P., Wahle K. W., Fletcher J. M. Tissue and whole-body protein synthesis in immature Zucker rats and their relationship to protein deposition. Biochem J. 1982 May 15;204(2):393–398. doi: 10.1042/bj2040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E. A., Mezey E., Halsted C. H., Schuster M. M. Effect of ethanol on motility of the small intestine. Johns Hopkins Med J. 1974 Jul;135(1):17–24. [PubMed] [Google Scholar]
- Roggin G. M., Iber F. L., Kater R. M., Tabon F. Malabsorption in the chronic alcoholic. Johns Hopkins Med J. 1969 Dec;125(6):321–330. [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Contrasting response of protein degradation to starvation and insulin as measured by release of N tau-methylhistidine or phenylalanine from the perfused rat heart. Biochem J. 1986 Jul 15;237(2):391–395. doi: 10.1042/bj2370391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Ward R. J., Peters T. J. Fatty acid synthesis and triacylglycerol accumulation in rat liver after chronic ethanol consumption. Clin Sci (Lond) 1987 Aug;73(2):159–163. doi: 10.1042/cs0730159. [DOI] [PubMed] [Google Scholar]


