Abstract
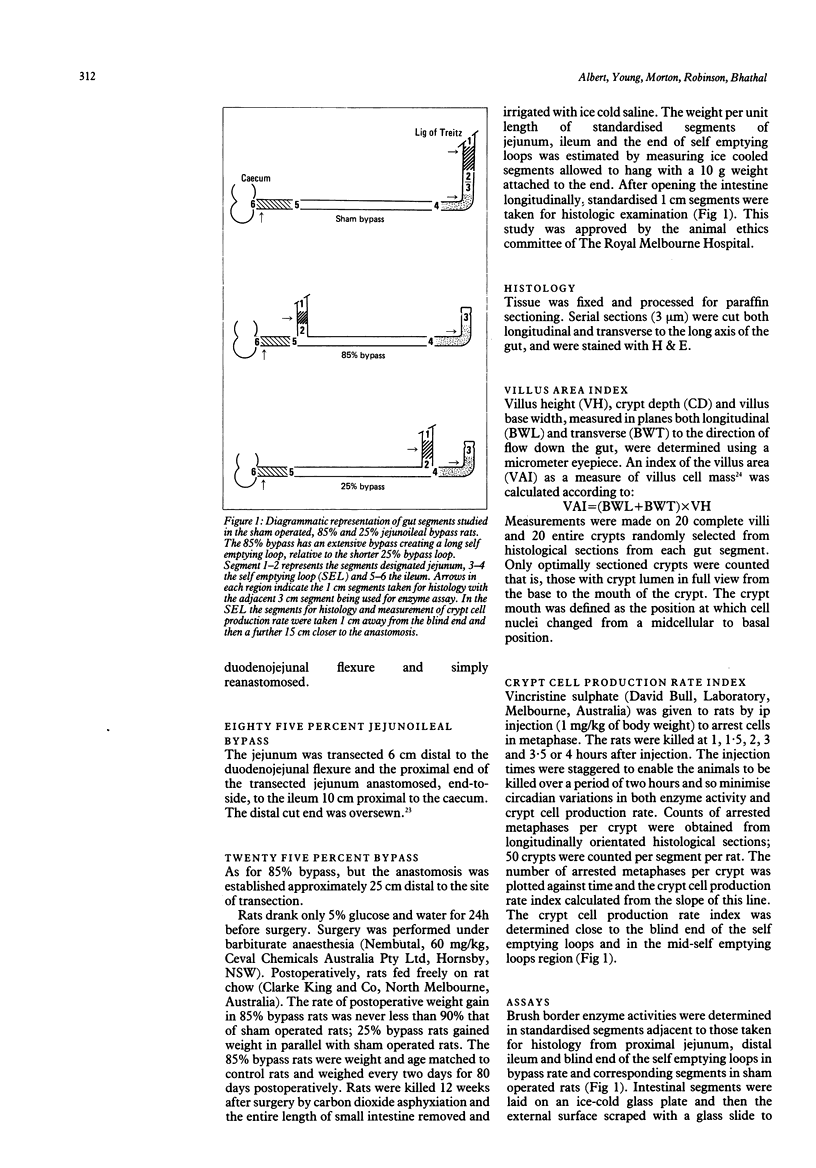
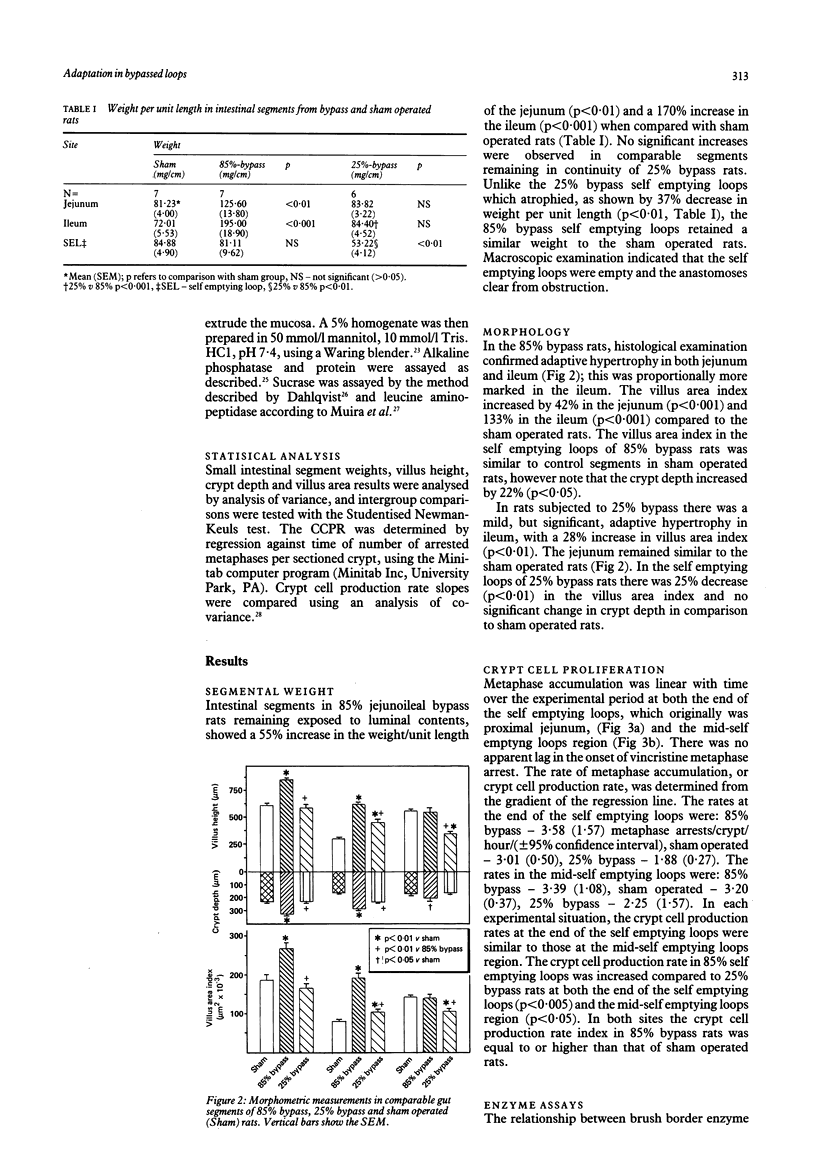
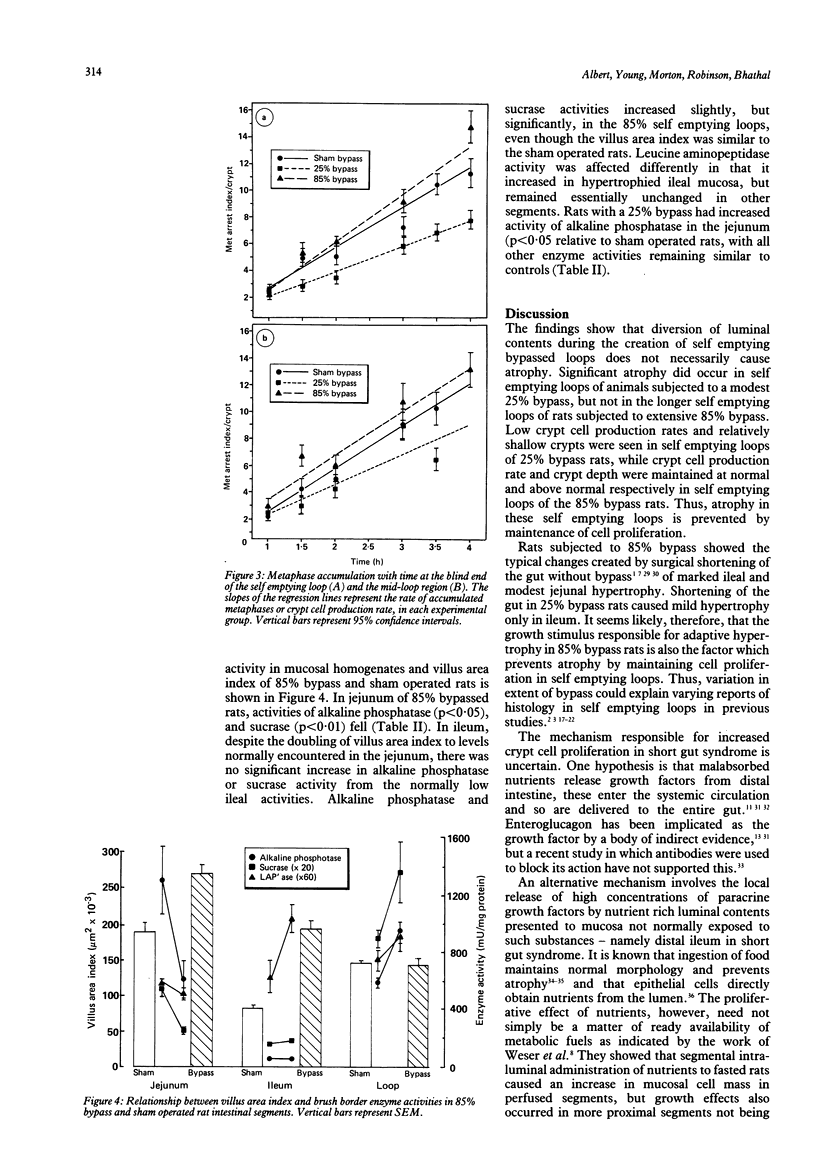
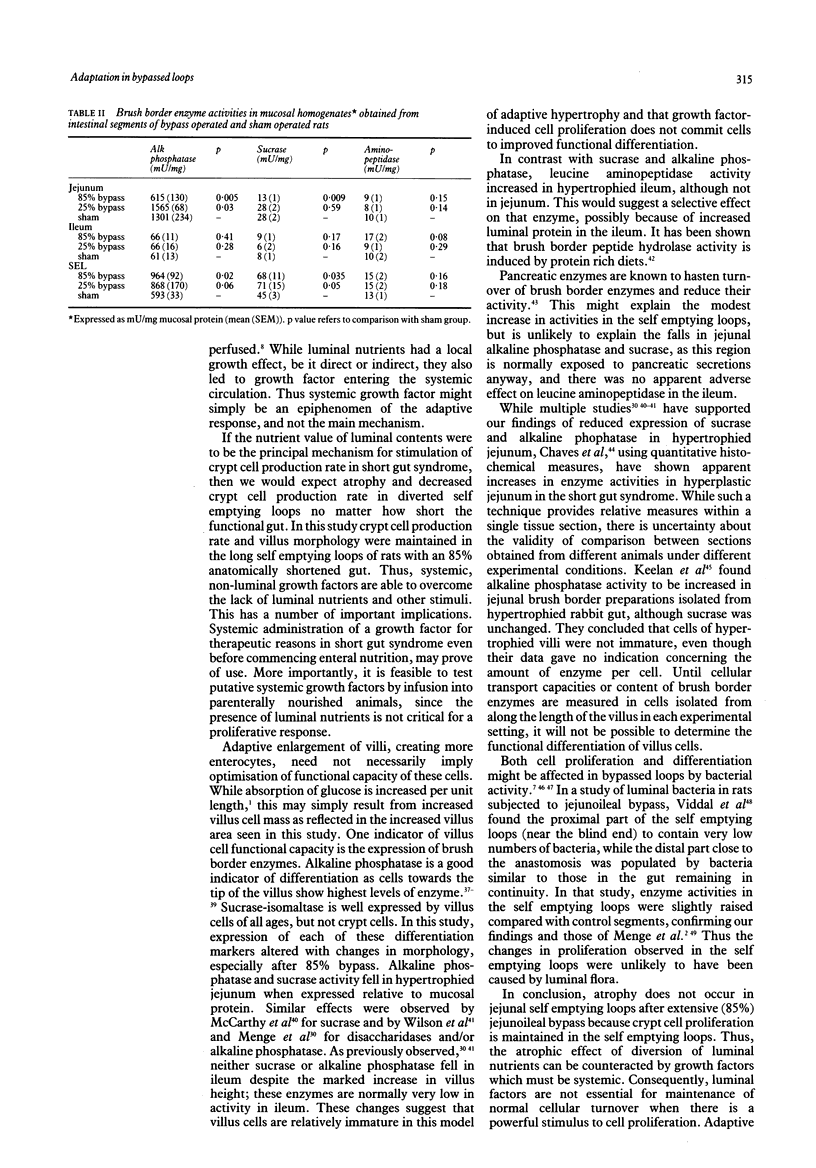
Mucosal histology, crypt cell proliferation and brush border enzymes were measured in rats with varying degrees of jejunoileal bypass, in order to compare the effect of systemic and luminal factors on adaptive growth and differentiation (brush border enzymes) in small intestinal epithelium. Eighty five percent jejunoileal bypass caused a functional short gut; in intestine remaining in continuity there were significant increases in segmental weight, villus area and crypt depth, compared with sham operated controls and 25% jejunoileal bypass rats. Despite villus cell hyperplasia in 85% bypass rats, mucosal sucrase and alkaline phosphatase fell in jejunum and remained low in ileum, while leucine amino peptidase rose in ileum. There was a significant fall in villus area (p less than 0.01) and crypt cell production (p less than 0.001) in self emptying loops of 25% bypass rats not exposed to luminal contents compared with control segments of sham operated rats. In contrast, self emptying loops of 85% bypass rats were not atrophied despite the much greater distance from luminal nutrients; the villus area (p less than 0.01) and crypt cell production (p less than 0.005) were higher than in 25% bypass rats, and at least as great as in sham operated rats. These results indicate that adaptive hyperplasia has a variable effect on expression of brush border enzymes which might reflect villus cell immaturity. The atrophic effect of diversion of luminal contents can be counteracted by systemic growth factors released as part of the adaptive response; thus systemic growth factors are not dependent on a permissive effect of luminal contents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jurf A. S., Younoszai M. K., Chapman-Furr F. Effect of nutritional method on adaptation of the intestinal remnant after massive bowel resection. J Pediatr Gastroenterol Nutr. 1985 Apr;4(2):245–252. doi: 10.1097/00005176-198504000-00016. [DOI] [PubMed] [Google Scholar]
- Alpers D. H. Protein synthesis in intestinal mucosa: the effect of route of administration of precursor amino acids. J Clin Invest. 1972 Jan;51(1):167–173. doi: 10.1172/JCI106788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H., Tedesco F. J. The possible role of pancreatic proteases in the turnover of intestinal brush border proteins. Biochim Biophys Acta. 1975 Aug 5;401(1):28–40. doi: 10.1016/0005-2736(75)90338-7. [DOI] [PubMed] [Google Scholar]
- Altmann G. G. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. Am J Anat. 1971 Oct;132(2):167–177. doi: 10.1002/aja.1001320204. [DOI] [PubMed] [Google Scholar]
- Altmann G. G. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat. 1972 Apr;133(4):391–400. doi: 10.1002/aja.1001330403. [DOI] [PubMed] [Google Scholar]
- Besterman H. S., Adrian T. E., Mallinson C. N., Christofides N. D., Sarson D. L., Pera A., Lombardo L., Modigliani R., Bloom S. R. Gut hormone release after intestinal resection. Gut. 1982 Oct;23(10):854–861. doi: 10.1136/gut.23.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller J. A., Montgomery R. K., Grand R. J., Klagsbrun M., Rosenthal A. Use of a 3T3 cell growth factor assay for the delineation and characterization of humoral trophic factors involved in intestinal adaptation in the rat. Gastroenterology. 1986 Aug;91(2):448–455. doi: 10.1016/0016-5085(86)90581-0. [DOI] [PubMed] [Google Scholar]
- Bloom S. R. Gut hormones in adaptation. Gut. 1987;28 (Suppl):31–35. doi: 10.1136/gut.28.suppl.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves M., Smith M. W., Williamson R. C. Increased activity of digestive enzymes in ileal enterocytes adapting to proximal small bowel resection. Gut. 1987 Aug;28(8):981–987. doi: 10.1136/gut.28.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M. Control of intestinal epithelial replacement: lack of evidence for a tissue-specific blood-borne factor. Cell Tissue Kinet. 1974 May;7(3):241–250. doi: 10.1111/j.1365-2184.1974.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Feldman E. J., Dowling R. H., McNaughton J., Peters T. J. Effects of oral versus intravenous nutrition on intestinal adaptation after small bowel resection in the dog. Gastroenterology. 1976 May;70(5 PT1):712–719. [PubMed] [Google Scholar]
- Fenyö G., Hallberg D. Intestinal hypertrophy after small intestinal bypass in the rat. Studies on methods and reversibility of changes. Acta Chir Scand. 1976;142(3):261–269. [PubMed] [Google Scholar]
- Gleeson M. H., Cullen J., Dowling R. H. Intestinal structure and function after small bowel by-pass in the rat. Clin Sci. 1972 Dec;43(6):731–742. doi: 10.1042/cs0430731. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Wilson T. J., Lenton W., Gregory H., McCullagh K. G., Wright N. A. Proliferative effects of urogastrone-EGF on the intestinal epithelium. Gut. 1987;28 (Suppl):37–43. doi: 10.1136/gut.28.suppl.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M., Menge H., Stössel R., Riecken E. O. Effect of monoclonal antibodies to enteroglucagon on ileal adaptation after proximal small bowel resection. Gut. 1987;28 (Suppl):9–14. doi: 10.1136/gut.28.suppl.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A., Krishnan C., Forstner G. Pathogenesis of mucosal injury in the blind loop syndrome. Gastroenterology. 1978 Nov;75(5):791–795. [PubMed] [Google Scholar]
- Keelan M., Walker K., Thomson A. B. Resection of rabbit ileum: effect on brush border membrane enzyme markers and lipids. Can J Physiol Pharmacol. 1985 Dec;63(12):1528–1532. doi: 10.1139/y85-251. [DOI] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Li A. K., Jeppsson B. W., Jamieson C. G. Intraluminal versus humoral factors in intestinal cell proliferation. Br J Surg. 1982 Oct;69(10):569–572. doi: 10.1002/bjs.1800691004. [DOI] [PubMed] [Google Scholar]
- McCarthy D. M., Kim Y. S. Changes in sucrase, enterokinase, and peptide hydrolase after intestinal resection. The association of cellular hyperplasia and adaptation. J Clin Invest. 1973 Apr;52(4):942–951. doi: 10.1172/JCI107259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge H., Bloch R., Schaumlöffel E., Riecken E. O. Transportstudien, morphologische, morphometrische und histochemische Untersuchungen zum Verhalten der Dünndarmschleimhaut im operativ ausgeschalteten Jejunalabschnitt der Ratte. Z Gesamte Exp Med. 1970;153(1):74–90. [PubMed] [Google Scholar]
- Menge H., Robinson J. W., Schroeder P. Functional and structural correlations in the atrophic mucosa of self-emptying blind loops of rat small intestine [proceedings]. J Physiol. 1978 Jul;280:33P–34P. [PubMed] [Google Scholar]
- Menge H., Robinson J. W. The relationship between the functional and structural alterations in the rat small intestine following proximal resection of varying extents. Res Exp Med (Berl) 1978 Jul 24;173(1):41–53. doi: 10.1007/BF01851373. [DOI] [PubMed] [Google Scholar]
- Miura S., Morita A., Erickson R. H., Kim Y. S. Content and turnover of rat intestinal microvillus membrane aminopeptidase. Effect of methylprednisolone. Gastroenterology. 1983 Dec;85(6):1340–1349. [PubMed] [Google Scholar]
- Sagor G. R., Ghatei M. A., Al-Mukhtar M. Y., Wright N. A., Bloom S. R. Evidence for a humoral mechanism after small intestinal resection. Exclusion of gastrin but not enteroglucagon. Gastroenterology. 1983 May;84(5 Pt 1):902–906. [PubMed] [Google Scholar]
- Tilson M. D., Wright H. K. Adaptation of functioning and bypassed segments of ileum during compensatory hypertrophy of the gut. Surgery. 1970 Apr;67(4):687–693. [PubMed] [Google Scholar]
- Ulshen M. H., Lyn-Cook L. E., Raasch R. H. Effects of intraluminal epidermal growth factor on mucosal proliferation in the small intestine of adult rats. Gastroenterology. 1986 Nov;91(5):1134–1140. doi: 10.1016/s0016-5085(86)80008-7. [DOI] [PubMed] [Google Scholar]
- Van Dongen J. M., Kooyman J., Visser W. J., Holt S. J., Galjaard H. The effect of increased crypt cell proliferation on the activity and subcellular localization of esterases and alkaline phosphatase in the rat small intestine. Histochem J. 1977 Jan;9(1):61–75. doi: 10.1007/BF01007009. [DOI] [PubMed] [Google Scholar]
- Viddal K. O., Midtvedt T., Nygaard K. Intestinal bypass. Bacteriological studies from different parts of the small intestine in rats. Scand J Gastroenterol. 1983 Jul;18(5):619–625. doi: 10.3109/00365528309181648. [DOI] [PubMed] [Google Scholar]
- Weser E., Babbitt J., Hoban M., Vandeventer A. Intestinal adaptation. Different growth responses to disaccharides compared with monosaccharides in rat small bowel. Gastroenterology. 1986 Dec;91(6):1521–1527. [PubMed] [Google Scholar]
- Weser E. Luminal nutrients and intestinal adaptation. J Pediatr Gastroenterol Nutr. 1985 Apr;4(2):165–166. doi: 10.1097/00005176-198504000-00002. [DOI] [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Malt R. A. Proximal enterectomy stimulates distal hyperplasia more than bypass or pancreaticobiliary diversion. Gastroenterology. 1978 Jan;74(1):16–23. [PubMed] [Google Scholar]
- Williamson R. C., Buchholtz T. W., Malt R. A. Humoral stimulation of cell proliferation in small bowel after transection and resection in rats. Gastroenterology. 1978 Aug;75(2):249–254. [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Irwin M. The kinetics of villus cell populations in the mouse small intestine. I. Normal villi: the steady state requirement. Cell Tissue Kinet. 1982 Nov;15(6):595–609. doi: 10.1111/j.1365-2184.1982.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Young G. P., Morton C. L., Rose I. S., Taranto T. M., Bhathal P. S. Effects of intestinal adaptation on insulin binding to villus cell membranes. Gut. 1987;28 (Suppl):57–62. doi: 10.1136/gut.28.suppl.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. P., Yedlin S. T., Alpers D. H. Distribution of soluble and membranous forms of alkaline phosphatase in the small intestine of the rat. Biochim Biophys Acta. 1981 Aug 17;676(2):257–265. doi: 10.1016/0304-4165(81)90194-x. [DOI] [PubMed] [Google Scholar]
- de Both N. J., Plaisier H. The influence of changing cell kinetics on functional differentiation in the small intestine of the rat. A study of enzymes involved in carbohydrate metabolism. J Histochem Cytochem. 1974 May;22(5):352–360. doi: 10.1177/22.5.352. [DOI] [PubMed] [Google Scholar]


