Abstract
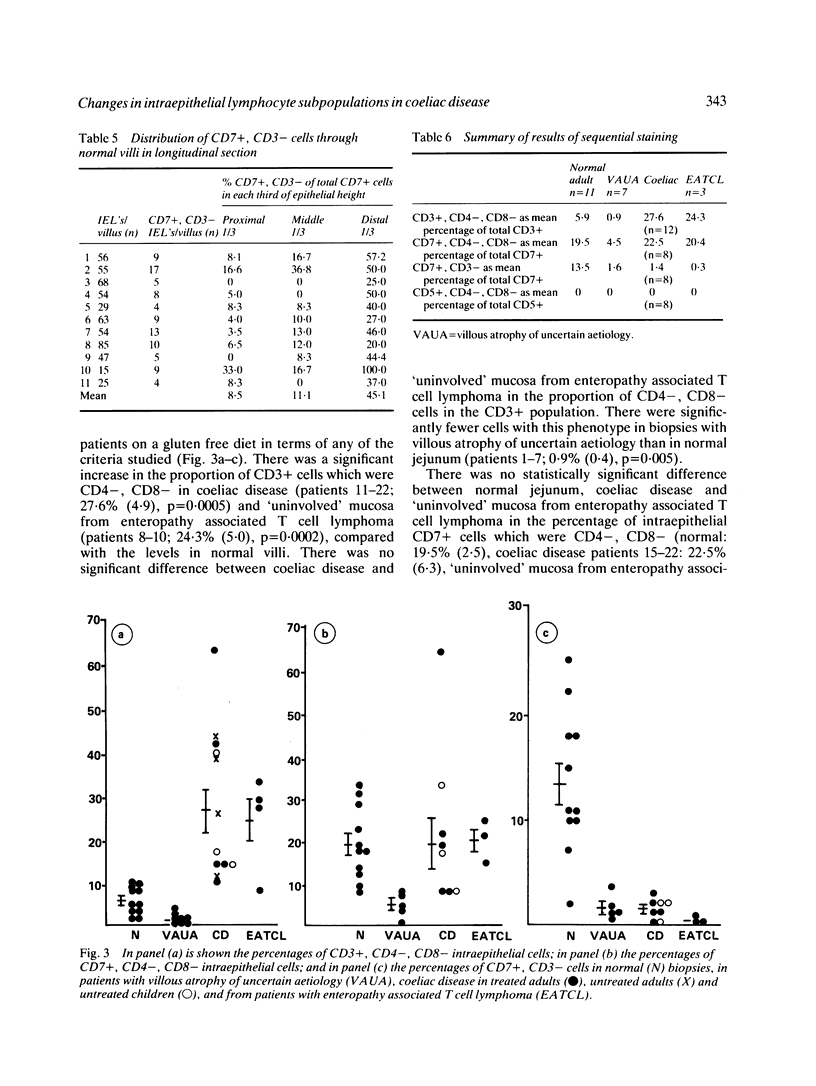
Studies of the morphologic and phenotypic diversity of intraepithelial T cells in human small intestine have shown them to be heterogeneous, yet distinct from most extra intestinal T cells. In this study sequential immunoenzymatic staining was used to define new intraepithelial lymphocyte subpopulations in man. In normal human jejunum approximately 6% of the intraepithelial T cells expressing CD3 (an antigen associated with the T cell receptor) do not express the T cell subset antigens CD4 or CD8. Approximately 20% of CD7+ cells (T cells and null cells) do not express CD4 or CD8 and 14% of the CD7+ cells do not express CD3 and are therefore not T cells. The CD7+, CD3+/-, CD4-, CD8- population is concentrated in the tips of the villi. In coeliac disease, the ratios of the subsets change significantly. The percentage of CD3+, 4-, 8- cells increases to 28%, the proportion of CD7+, 4-, 8- cells remains unchanged and the CD7+, CD3- (non-T cell) population is reduced to 1.4% of the CD7+ cells. In contrast, in patients with villous atrophy of uncertain aetiology, all CD4-, CD8- lymphocyte subsets are decreased compared with normal biopsies. Finally, in enteropathy associated T cell lymphoma (malignant histiocytosis of the intestine) in which the 'uninvolved mucosa' is histologically similar to untreated coeliac disease, the changes in the intraepithelial T cell sub-sets are indistinguishable from those in coeliac disease, suggesting that the lymphoma is a complication of coeliac disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank I., DePinho R. A., Brenner M. B., Cassimeris J., Alt F. W., Chess L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature. 1986 Jul 10;322(6075):179–181. doi: 10.1038/322179a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- EAKINS D., FULTON T., HADDEN D. R. RETICULUM CELL SARCOMA OF THE SMALL BOWEL AND STEATORRHOEA. Gut. 1964 Aug;5:315–323. doi: 10.1136/gut.5.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson P. G., O'Connor N. T., Spencer J., Bevan D. H., Connolly C. E., Kirkham N., Pollock D. J., Wainscoat J. S., Stein H., Mason D. Y. Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet. 1985 Sep 28;2(8457):688–691. doi: 10.1016/s0140-6736(85)92930-7. [DOI] [PubMed] [Google Scholar]
- Isaacson P., Wright D. H. Intestinal lymphoma associated with malabsorption. Lancet. 1978 Jan 14;1(8055):67–70. doi: 10.1016/s0140-6736(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Jenkins D., Goodall A., Scott B. B. T-lymphocyte populations in normal and coeliac small intestinal mucosa defined by monoclonal antibodies. Gut. 1986 Nov;27(11):1330–1337. doi: 10.1136/gut.27.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E. Y., Lanier L. L., Turck C. W., Littman D. R., Davis M. M., Chien Y. H., Weiss A. Identification and sequence of a fourth human T cell antigen receptor chain. Nature. 1987 Dec 10;330(6148):569–572. doi: 10.1038/330569a0. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology. 1980 Sep;79(3):481–492. [PubMed] [Google Scholar]
- Moretta L., Pende D., Bottino C., Migone N., Ciccone E., Ferrini S., Mingari M. C., Moretta A. Human CD3+4-8-WT31- T lymphocyte populations expressing the putative T cell receptor gamma-gene product. A limiting dilution and clonal analysis. Eur J Immunol. 1987 Sep;17(9):1229–1234. doi: 10.1002/eji.1830170903. [DOI] [PubMed] [Google Scholar]
- O'Driscoll B. R., Stevens F. M., O'Gorman T. A., Finnegan P., McWeeney J. J., Little M. P., Connolly C. E., McCarthy C. F. HLA type of patients with coeliac disease and malignancy in the west of Ireland. Gut. 1982 Aug;23(8):662–665. doi: 10.1136/gut.23.8.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrelly C., Feighery C., O'Briain D. S., Stevens F., Connolly C. E., McCarthy C., Weir D. G. Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma. Br Med J (Clin Res Ed) 1986 Oct 11;293(6552):908–910. doi: 10.1136/bmj.293.6552.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. M., Krajewski A. S., Dewar A. E. Immunophenotype analysis of malignant histiocytosis of the intestine. J Clin Pathol. 1986 Jan;39(1):8–15. doi: 10.1136/jcp.39.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. M., Krajewski A. S., Kelleher D. Histogenesis of malignant histiocytosis of the intestine. Gastroenterology. 1987 Jun;92(6):2050–2051. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Cerf-Bensussan N., Jarry A., Brousse N., Guy-Grand D., Krajewski A. S., Isaacson P. G. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1988 Jul;132(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]



