Abstract
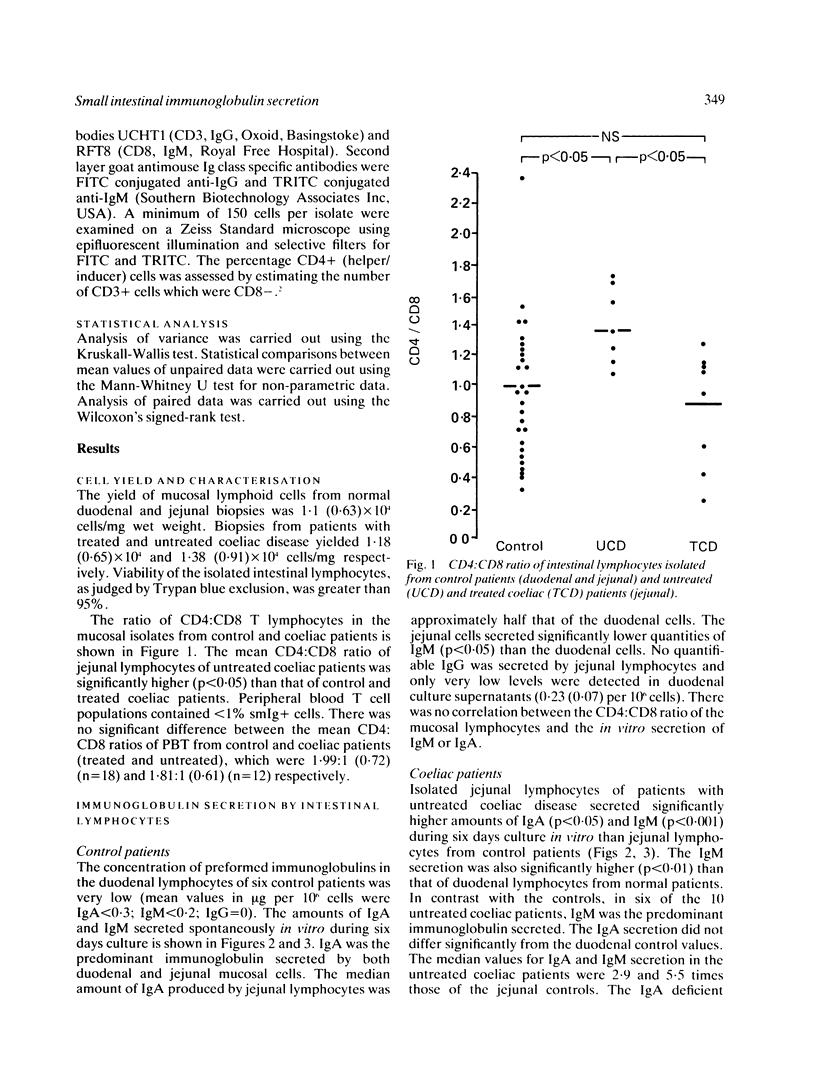
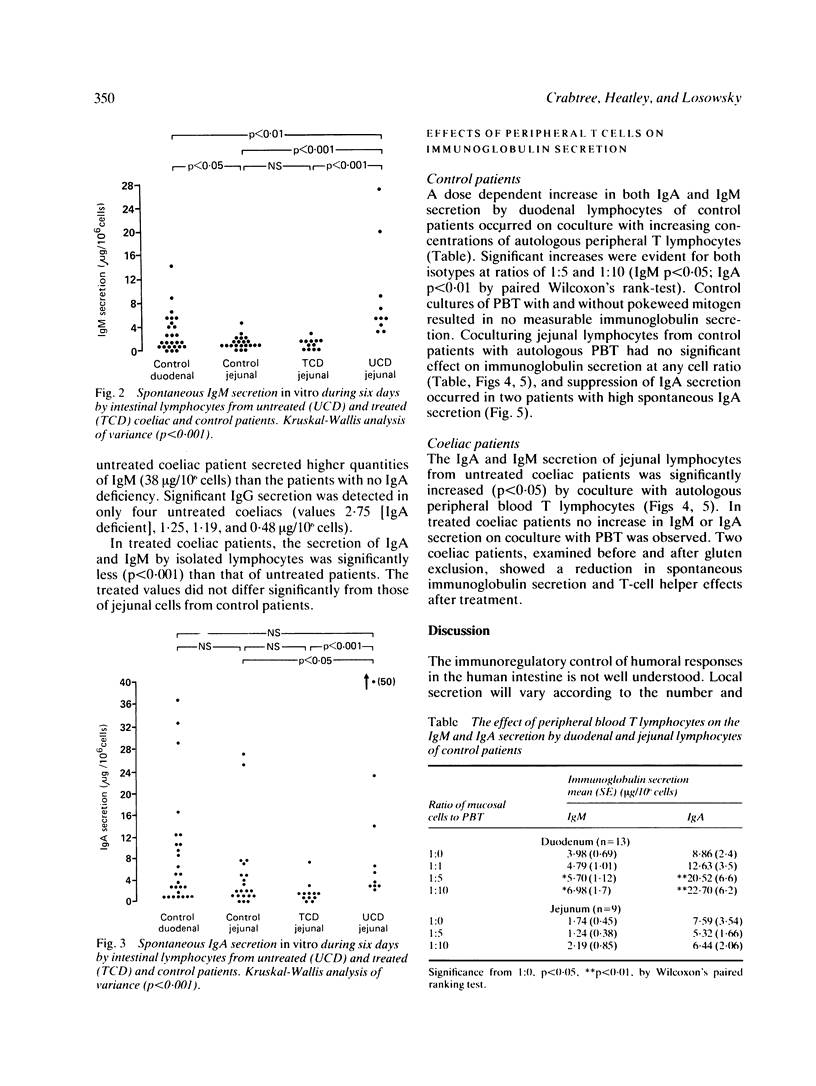
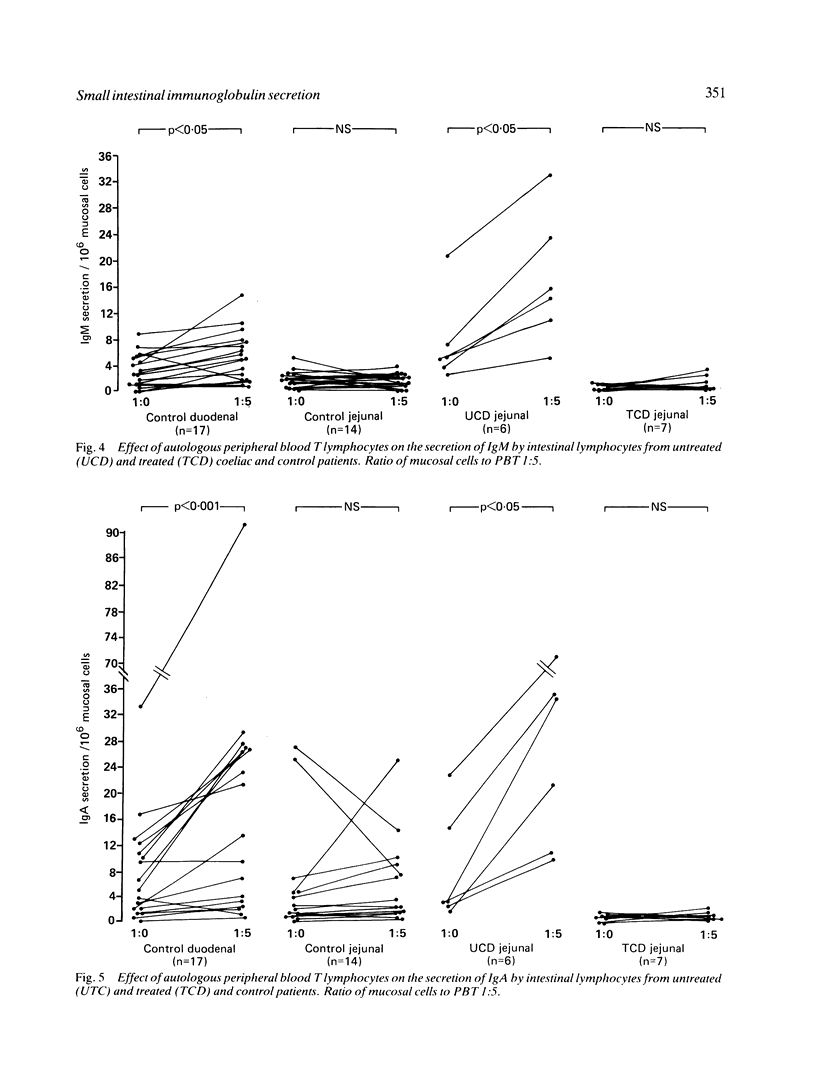
The in vitro secretion of immunoglobulins by small intestinal lymphocytes isolated from 47 patients with normal histology and 23 patients with treated and untreated coeliac disease was examined using an enzyme linked immunosorbent assay. In control patients, duodenal lymphocytes spontaneously secreted higher levels of IgM than jejunal lymphocytes (p less than 0.05). Significantly higher levels of both IgA (p less than 0.05) and IgM (p less than 0.001) were secreted by jejunal lymphocytes of 10 patients with untreated coeliac disease than cells isolated from normal jejunal tissue. IgM and IgA secretion by duodenal lymphocytes isolated from control patients was increased in a dose dependent manner by coculture with autologous peripheral blood T lymphocytes. This effect was not observed with jejunal lymphocytes of control or treated coeliac patients. Peripheral T-cells of untreated coeliac patients, however, showed significant helper effects (p less than 0.05) for IgM and IgA secretion by autologous jejunal lymphocytes. The results suggest that jejunal lymphocytes of patients with untreated coeliac disease show major differences in their capacity to synthesise and secrete immunoglobulins in vitro and the enhanced secretion might result from changes in T-cell immunoregulatory function.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baklien K., Brandtzaeg P., Fausa O. Immunoglobulins in jejunal mucosa and serum from patients with adult coeliac disease. Scand J Gastroenterol. 1977;12(2):149–159. [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton R. W., Cochrane C., McClelland D. B. Preparation of lymphoid cells from small specimens of human gastrointestinal mucosa. Gut. 1978 Oct;19(10):898–906. doi: 10.1136/gut.19.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis V. A., Heatley R. V. Evidence for regulation of human colonic mucosal immunoglobulin secretion by intestinal lymphoid cells. J Clin Lab Immunol. 1987 Jan;22(1):7–11. [PubMed] [Google Scholar]
- Dhesi I., Marsh M. N., Kelly C., Crowe P. Morphometric analysis of small intestinal mucosa. II. Determination of lamina propria volumes; plasma cell and neutrophil populations within control and coeliac disease mucosae. Virchows Arch A Pathol Anat Histopathol. 1984;403(2):173–180. doi: 10.1007/BF00695233. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Weiserbs D. B., Ealding W., Machelski E. T-helper cell activity in intestinal lamina propria. Ann N Y Acad Sci. 1983 Jun 30;409:230–237. doi: 10.1111/j.1749-6632.1983.tb26872.x. [DOI] [PubMed] [Google Scholar]
- Fluge G., Aksnes L. Quantification of immunoglobulins after organ culture of human duodenal mucosa. J Pediatr Gastroenterol Nutr. 1983;2(1):62–70. doi: 10.1097/00005176-198302010-00008. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Eardley D. D., Durum S., Green D. R., Shen F. W., Yamauchi K., Cantor H., Murphy D. B. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981 Jun 1;153(6):1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Gold J., St Martin S., Gershon R., Gershon R. K. Microenvironmental immunoregulation: possible role of contrasuppressor cells in maintaining immune responses in gut-associated lymphoid tissues. Proc Natl Acad Sci U S A. 1982 Feb;79(3):889–892. doi: 10.1073/pnas.79.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. Regulation of human B lymphocyte activation, proliferation, and differentiation. Adv Immunol. 1987;40:1–59. doi: 10.1016/s0065-2776(08)60237-0. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J., O'Farrelly C., O'Mahony C., Weir D. G., Feighery C. Immunoperoxidase demonstration of the cellular composition of the normal and coeliac small bowel. Clin Exp Immunol. 1987 Apr;68(1):177–188. [PMC free article] [PubMed] [Google Scholar]
- Lancaster-Smith M., Kumar P., Marks R., Clark M. L., Dawson A. M. Jejunal mucosal immunoglobulin-containing cells and jejunal fluid immunoglobulins in adult coeliac disease and dermatitis herpetiformis. Gut. 1974 May;15(5):371–376. doi: 10.1136/gut.15.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Avery J., Jones T. Separation and characterization of a subset of human T8+ cells which function as antigen-presenting and contrasuppressor cells. Immunology. 1985 Apr;54(4):713–722. [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Mohrman R. F., Kodner I. J., Delacroix D. L., Vaerman J. P. Altered patterns of secretion of monomeric IgA and IgA subclass 1 by intestinal mononuclear cells in inflammatory bowel disease. Gastroenterology. 1986 Aug;91(2):379–385. doi: 10.1016/0016-5085(86)90572-x. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Seiden M. V., Bragdon M. J., Beale M. G. Alterations of IgM, IgG, and IgA Synthesis and secretion by peripheral blood and intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Gastroenterology. 1981 Nov;81(5):844–852. [PubMed] [Google Scholar]
- Malizia G., Trejdosiewicz L. K., Wood G. M., Howdle P. D., Janossy G., Losowsky M. S. The microenvironment of coeliac disease: T cell phenotypes and expression of the T2 'T blast' antigen by small bowel lymphocytes. Clin Exp Immunol. 1985 May;60(2):437–446. [PMC free article] [PubMed] [Google Scholar]
- Scott B. B., Scott D. G., Losowsky M. S. Jejunal mucosal immunoglobulins and complement in untreated coeliac disease. J Pathol. 1977 Apr;121(4):219–223. doi: 10.1002/path.1711210405. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Lymphocyte subpopulations in the human small intestine. The findings in normal mucosa and in the mucosa of patients with adult coeliac disease. Clin Exp Immunol. 1983 Apr;52(1):219–228. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Jewell D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981 Mar;22(3):169–176. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Williams N., Adams P. The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods. 1972 May;1(3):273–287. doi: 10.1016/0022-1759(72)90005-1. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Chess L. Relationship between human T cell functional heterogeneity and human T cell surface molecules. Immunol Rev. 1983;74:113–128. doi: 10.1111/j.1600-065x.1983.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Wood G. M., Howdle P. D., Trejdosiewicz L. K., Losowsky M. S. Jejunal plasma cells and in vitro immunoglobulin production in adult coeliac disease. Clin Exp Immunol. 1987 Jul;69(1):123–132. [PMC free article] [PubMed] [Google Scholar]
- Wood G. M., Shires S., Howdle P. D., Losowsky M. S. Immunoglobulin production by coeliac biopsies in organ culture. Gut. 1986 Oct;27(10):1151–1160. doi: 10.1136/gut.27.10.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. M., Trejdosiewicz L. K., Losowsky M. S. ELISA for measurement of secretory IgA distinct from monomeric IgA. J Immunol Methods. 1987 Mar 12;97(2):269–274. doi: 10.1016/0022-1759(87)90470-4. [DOI] [PubMed] [Google Scholar]


