Abstract
Trypanosomatids, the etiologic agents of sleeping sickness, leishmaniasis, and Chagas' disease, compartmentalize glycolysis within glycosomes, metabolic organelles related to peroxisomes. Here, we identify a trypanosome homologue of PEX14, one of the components of the peroxisomal protein import docking complex. We have used double-stranded RNA interference to target the PEX14 transcript for degradation. Glycosomal matrix protein import was compromised, and both glycolytic bloodstream stage parasites and mitochondrially respiring procyclic stage parasites were killed. Thus, unlike peroxisomes, glycosomes are essential organelles. Surprisingly, procyclic forms, which can grow in the absence of glucose, were killed by PEX14 RNA interference only when simple sugars were present. Thus, interference with glycosome protein import makes glucose toxic to trypanosomes.
The protozoan parasite Trypanosoma brucei is the causative agent of bovine nagana and human sleeping sickness, a fatal disease estimated to affect 300,000–500,000 persons per year (www.who.int/inf-fs/en/fact259.html). T. brucei and related parasites are the only organisms known to compartmentalize glycolysis within an organelle. This organelle, the glycosome, is evolutionarily related to peroxisomes, as shown by the commonality of certain metabolic functions, targeting sequences, and biogenesis proteins (1).
Each parasite possesses numerous glycosomes that house not only glycolysis (2) but also enzymes of ether-lipid biosynthesis (3, 4), β-oxidation of fatty acids (5), and purine salvage (6). The spectrum of proteins contained within the glycosome changes during parasite development. Glycolytic enzymes are reduced in abundance in the insect midgut (procyclic) stage (7), which generates ATP through cytochrome-mediated respiration. In contrast, glycolytic enzymes are very abundant in mammalian bloodstream stage parasites, which rely solely on glycolysis and substrate-level phosphorylation for the generation of energy (7). Thus, the glycosome and its constituents are considered potential targets for therapies to combat sleeping sickness (8, 9).
Recently, proteins considered to function in glycosomal protein import have been identified, and these show significant similarities to those demonstrated to function in peroxisomal protein import in yeast and mammals. Among such proteins are PEX5, the receptor for the type 1 peroxisomal targeting sequence (10, 11), and PEX2 (12), a protein required for peroxisomal protein import but whose exact function remains elusive. Also identified is a homologue of PEX11 (13), a peroxisomal membrane protein thought to function in fatty acid metabolism (14) as well as regulation of peroxisomal abundance (15). In previous studies, it was not possible to knock out the Leishmania donovani PEX2 gene (16) or the T. brucei PEX11 gene (13), suggesting that they are essential.
The current work focuses on the trypanosome homologue of PEX14, a peroxisomal membrane protein whose only known function is to directly participate in the import of matrix proteins (17–19). Like other PEX genes, PEX14 is not essential for viability of yeast cells (19, 20). In this report, we identify the T. brucei homologue of PEX14 and use double-stranded RNA interference (RNAi) to demonstrate that disruption of PEX14 function is toxic to both bloodstream and procyclic form parasites. Surprisingly, removal of simple sugars from the medium protected procyclic form parasites from PEX14 RNAi.
Materials and Methods
Plasmid Construction.
Nucleotides 17–418 of the TbPEX14 ORF were amplified through PCR from T. brucei genomic DNA with Pfu DNA polymerase (Stratagene). The primers (5′-TGAATCTCGAGCGGGGGTAGTGGATGACGGC-3′ and 5′-GCGGCAAGCTTGGCAGCAGCCGCTTCGGGG-3′) add XhoI and HindIII sites for cloning into RNAi vector pZJM (21) digested with the same enzymes. The resulting plasmid contains the phleomycin-resistance marker plus the TbPEX14 insert flanked on either side by convergent tetracycline (Tet) operator-controlled T7 promoters.
Expression of TbPEX14 and Generation of Antibodies.
A fragment encoding amino acids 1–171 of TbPEX14 was amplified from T. brucei EATRO 110 genomic DNA by using Pfu DNA polymerase, and primers (5′-ATCTCCCATGGCTTTGCTGCTGTCGG-3′ and 5′-ATGGATCCATTTCATAAGGTGAATACG-3′), adding NcoI and BamHI sites to the 5′ and 3′ ends, respectively (underlined). The fragment was dA-tailed with Taq DNA polymerase, cloned into the Topo-TA vector (Invitrogen), sequenced, and subcloned into NcoI/BamHI-cleaved pET 30b (+). The resulting construct specifies a TbPEX14 fusion protein containing an N-terminal hexahistidine (His6-tag) and the S-peptide of pancreatic RNase (S-tag) (Novagen). BL21 Escherichia coli cultures (1 liter) containing the plasmid were grown to an OD600 of 0.5 in Luria broth with 50 μg/ml kanamycin. After a 4-h induction at 37°C with 1 mM isopropyl β-D-thiogalactopyranoside, harvested cell pellets were resuspended in 20 ml of PBS containing EDTA-free mini tab protease inhibitor mixture (Roche Molecular Biochemicals). Cells were lysed by French press, and the His6/S-TbPEX14 was purified to homogeneity from the soluble fraction by affinity chromatography on an Ni2+-NTA matrix (Qiagen, Valencia, CA). The resulting protein was used to generate a rabbit polyclonal antiserum (Cocalico Biologicals, Reamstown, PA). To purify the antibodies, an affinity matrix was prepared by coupling 2 mg of His6/S-TbPEX14 to 1 ml of Affi-Gel 10 resin (Bio-Rad). Immune serum (2.0 ml) was diluted 2-fold with PBS and incubated with the affinity matrix for 4 h at 4°C. His6/S-TbPEX14 antibodies were eluted with 100 mM glycine, pH 2.5, and eluates were neutralized with 0.1 vol of 2.0 M Tris⋅HCl, pH 8.0.
Immunoblotting.
For purification of glycosomes, the postnuclear supernatant was subjected to centrifugation at 48,000 × g. The organellar pellet was resuspended and separated on a sucrose step gradient as described (22). Digitonin-separated fractions (23), or glycosomes fractionated with carbonate extraction (24), were subjected to SDS/polyacrylamide gel electrophoresis (10% acrylamide). Samples were transferred to nitrocellulose. For immunoblots, antisera directed against T. brucei PEX14 (1/500), phosphoglycerate kinase (PGK, 1/2,000) (25), Trypanosoma cruzi ribosomal protein P0 (1/2,500) (26), and Leishmania braziliensis mitochondrial HSP70 were used. Bound antibodies were revealed by using horseradish peroxidase-coupled goat anti-rabbit Ig (1:20,000) and chemiluminescent substrates (NEN ECL system).
Transfection and RNAi Induction.
T. brucei 29-13 procyclic forms (27) were grown in SDM-79 (28) and transfected by electroporation (23). After isolation of stable transfectants by selection using 2.5 μg/ml phleomycin, RNAi was induced by addition of Tet to 1 μg/ml. The cell concentration, assessed by using a Z1 Coulter counter, was initially 106 per ml and was kept between 5 × 105 and 5 × 106 per ml for the duration of the experiment. In some experiments, procyclic forms were grown in glucose-free medium. Glucosamine, glucose, NaHCO3, Mops, Hepes, and glycerol were deleted from SDM-79, and glucose-free RPMI medium 1640 was substituted for MEM and M199. Proline was added to 10 mM, and, as indicated, glucose was added to 25 mM. Dialyzed serum was used. Cells were adapted to the modified medium over a period of 2 weeks until they were in a mixture of 3 parts modified SDM-79/RPMI medium 1640 per 1 part standard SDM-79 (2.5 mM glucose). They were then placed in glucose-free SDM-79/RPMI medium 1640 for the experiments as described.
Bloodstream form T. brucei coexpressing T7 RNA polymerase and the Tet repressor (single marker cell line; ref. 27) were transfected as described (29) with modifications (30). After electroporation, cells were transferred to HMI-9 medium with G418 (31). Four phleomycin-resistant clones were obtained after about 2 weeks of selection with phleomycin (2.5 μg/ml). Induction of RNAi was conducted as described for procyclic forms, except that cell densities were kept between 1 × 105 and 4 × 106 per ml.
Viability was assessed by using the CellTiter 96 AQueous kit (Promega) and the LIVE/DEAD viability kit for animal cells (Molecular Probes).
Northern Analysis.
RNA was extracted by using Trizol (Invitrogen), and ≈10 μg of total RNA from each sample was separated by formaldehyde/agarose gel electrophoresis. After transfer to Nytran membranes, the blots were hybridized with an antisense RNA probe corresponding to the sequence in the PEX14 RNAi construct overnight at 65°C in 50% formamide. The blots were washed twice with 0.1× SSC (15 mM NaCl/1.5 mM sodium citrate, pH 7.0) and 0.1% SDS for 1 h at 65°C. Hybridizations using PEX5 (11), PEX7, and PGK (32) RNA probes were performed under the same conditions.
Immunofluorescence.
Cells were washed with PBS and placed on glass slides for 20 min. After fixation in 4% paraformaldehyde in PBS for 5 min, cells were permeabilized by 0.1% Triton X-100 in PBS for 5 min and blocked with 10% nonfat dry milk in PBS. The samples were incubated with antibodies in the blocking solution for 1 h, washed, and incubated with FITC-conjugated goat anti-rabbit IgG. After washing, slides were mounted in 50% glycerol in PBS for fluorescence microscopy.
Results
Identification of T. brucei PEX14.
A candidate TbPEX14 sequence was identified through database searching. The retrieved sequence contained a 1,098-nt ORF. TbPEX14 is most similar to other PEX14s in the N-terminal 70 aa, with dispersed short segments of similarity in other regions (Fig. 1A). PEX14s of other species possess a region of coiled coil structure, and the COILS program strongly predicts such a region at amino acids 220–320 of TbPEX14.
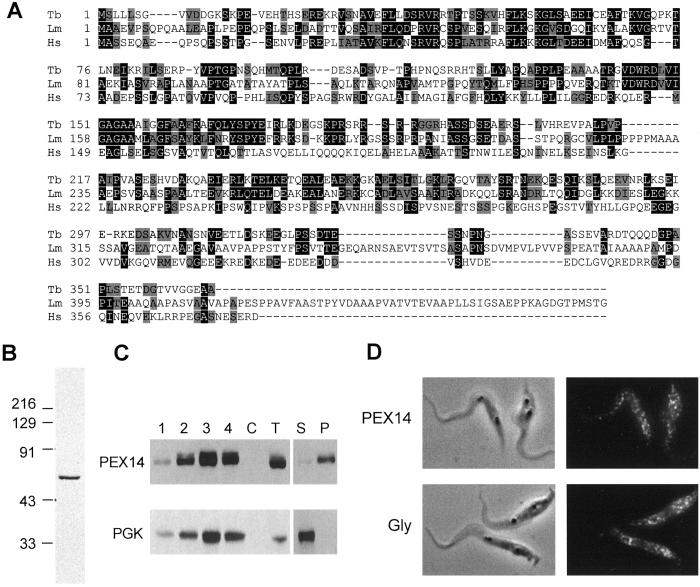
Fig 1.
Identification of TbPEX14. (A) Multiple sequence alignment. Human (XP_033058), T. brucei (AC087702), and Leishmania major (AL163505) PEX14 sequences were aligned using CLUSTALW. Identical residues are shaded black; conserved residues are shaded gray. (B) Immunoblot analysis of T. brucei procyclic form lysates by using affinity-purified anti-TbPEX14. (C) TbPEX14 fractionates as an integral glycosomal membrane protein. Lanes 1–4, sucrose gradient fractions of the 48,000 × g procyclic organellar pellet; C, cytosolic fraction generated by differential digitonin solubilization (106 cells); T, total cell lysate (2 × 106 procyclic forms). Gradient fraction 3 (20 μg) was extracted with 0.1 M sodium carbonate and centrifuged to separate the supernatant (S) containing matrix and peripheral membrane proteins from the membrane fraction (P, pellet). Blots were probed with anti-TbPEX14 or anti-PGK. The PGK blot shows the 56-kDa glycosomal isozyme. (D) Immunofluorescence with anti-PEX14 reveals a punctate pattern. Procyclic forms were stained with anti-glycosome or anti-TbPEX14, plus FITC-conjugated anti-rabbit IgG.
Given the modest level of sequence identity between the 366-aa T. brucei predicted protein and other PEX14s (19–24%), we performed localization studies to confirm its identity. Immunoblot analysis using an affinity-purified antiserum raised against a fragment of TbPEX14 detected a single species in procyclic form lysates (Fig. 1B). Like other PEX14s (17, 18, 33), this molecule migrates somewhat slower than its predicted mass (50 kDa apparent vs. 40 kDa predicted). The protein was not present in the cytosolic fraction upon digitonin solubilization (Fig. 1C, lane C), suggesting an organellar location. When the anti-PEX14 antiserum was used to probe fractions from a sucrose density gradient separating procyclic form organelles, PEX14 was specifically enriched in fractions 3 and 4, as was the glycosomal marker, the 56-kDa glycosomal PGK (Fig. 1C). Coomassie blue staining indicated that these same fractions showed the expected profile of proteins for procyclic form glycosomes (data not shown). Most PEX14 proteins are thought to be integral peroxisomal membrane proteins (17–19). The candidate TbPEX14 behaved as an integral membrane protein upon carbonate extraction (Fig. 1C), residing in the pellet fraction (lane P), whereas the glycosomal matrix PGK was found in the supernatant (lane S). Immunofluorescence analysis of procyclic (insect) stage parasites using the TbPEX14-specific antibodies revealed the punctate staining pattern characteristic of glycosomes (Fig. 1D). For comparison, the staining of glycosomes by using an anti-glycosomal antiserum that detects pyruvate phosphate dikinase, aldolase, and glyceraldehyde phosphate dehydrogenase (25) is shown. Together, these data demonstrate that the sequence identified encodes a T. brucei PEX14 homologue.
PEX14 RNAi in Procyclic Forms.
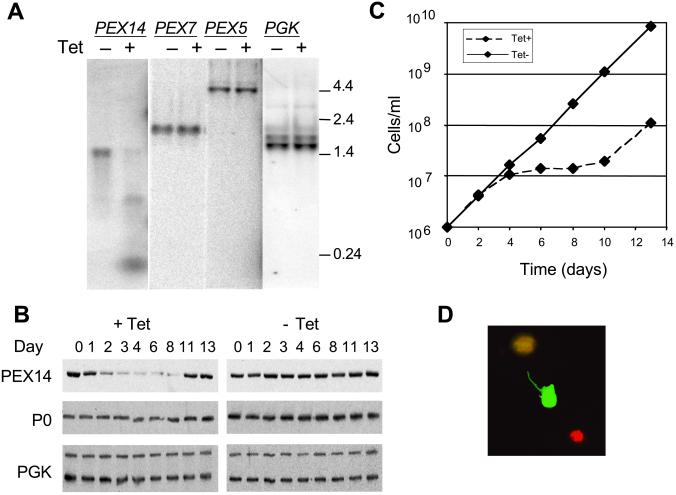
To disrupt the function of TbPEX14, a fragment of the coding sequence (nucleotides 17–418) was ligated into pZJM, a vector designed for Tet-regulated RNAi (21), and electroporated into T. brucei 29-13 procyclic forms (27). After the isolation of stable transfectants, expression of PEX14-specific double-stranded RNA (dsRNA) was induced by addition of Tet. Northern analysis showed that the amount of PEX14 mRNA was markedly decreased in the RNAi-induced cells by day 2 (Fig. 2A). Smaller hybridizing RNAs (≈400 bp and <200 bp) appeared, corresponding to the RNAi transcript and the expected small fragments produced by dsRNA-directed endonucleolytic cleavage. No alterations were observed in the abundance of selected other transcripts associated with glycosomal functions, e.g., PEX5, PEX7, and PGK (Fig. 2A). Reduced levels of TbPEX14 protein were noticeable by day 2 (Fig. 2B), and by day 4 the population growth slowed dramatically (Fig. 2C). On day 11, PEX14 levels increased and population growth resumed. This recovery may represent genetic or epigenetic escape from the regulatable RNAi system and occurred with clonal or nonclonal populations. Throughout the induction, there was no significant change in the levels of ribosomal protein P0 or the 56-kDa PGK (Fig. 2B).
Fig 2.
Induction of PEX14 RNAi in procyclic forms decreases PEX14 mRNA, protein, and cell viability. (A) Northern analysis. Total RNA was extracted from PEX14 RNAi cells after 2 days of culture with (+) or without (−) Tet (1 μg/ml), separated by formaldehyde gel electrophoresis, transferred to a nylon membrane, and probed with 32P-labeled riboprobes as indicated. (B) Immunoblot analysis. Parallel cultures induced (Tet+) or not induced (Tet−) were tested for abundance of TbPEX14, ribosomal protein P0, and the 56-kDa glycosomal and 45-kDa cytosolic PGK isozymes. (C) Growth and viability analysis. Shown is the cumulative cell number for parallel cultures grown in the presence or absence of Tet. (D) Live/dead staining of parasites on day 7 of the culture induced for RNAi. Dead cells fluoresce red because of uptake of ethidium homodimer 1, whereas live cells fluoresce green upon esterase activation of calcein acetoxymethyl ester.
PEX14 RNAi Affects Procyclic Parasite Viability.
To assess whether loss of PEX14 caused a static or killing effect, we measured the viability of the RNAi-induced population. On day 9, the RNAi-induced culture showed only ≈40% of the metabolic activity of the uninduced culture as revealed by bioreduction of a tetrazolium into formazan; ≈57% of cells were rounded and swollen in the induced culture as compared with <2% in uninduced cultures. Microscopic analysis (Fig. 2D) revealed that many of the abnormally shaped cells were dead as shown by their ability to take up ethidium homodimer and their lack of esterase activity. Thus, disruption of PEX14 function kills procyclic forms.
Removal of Glucose Protects Procyclic Forms from PEX14 RNAi.
As compartmentation of glycolysis is a unique feature of the glycosome, we tested the effect of glucose on parasites subjected to PEX14 RNAi (Fig. 3A). As expected, uninduced procyclic forms grown in proline-containing medium lacking glucose and other simple sugars continued to proliferate. Surprisingly, when PEX14 RNAi was induced, the cells continued to grow. Indeed, cultures without glucose grew at the same rate for 2 weeks whether or not PEX14 RNAi was induced. Seven days after the induction, parasites grown in the absence of glucose were supplemented with 25 mM glucose (Fig. 3B). These parasites began to round up within 24 h and doubled at most once, bypassing the 4- to 5-day phenotypic lag seen during induction of RNAi. Two days after the addition of glucose, 44% of the detectable parasites were dead as assessed by microscopic live/dead assay; by 4 days, 77% were dead.
Fig 3.
Glucose-mediated death in procyclic T. brucei PEX14 RNAi. RNAi was induced by addition of Tet to the concentration of 2 μg/ml. (A) Growth curve. Parasites were transferred to an RPMI 1640-based medium with or without glucose (+/−G). Tet was added to the indicated cultures (final concentration 2 μg/ml, +/−T), and growth was monitored. (B) Glucose add-back. After 7 days, the −glucose/+Tet culture shown in A was split, and parallel cultures were grown in the presence of Tet with or without glucose. (C) Immunofluorescence shows mislocalization of glycosomal proteins upon PEX14 RNAi. Procyclic forms were grown 7 days in glucose-free medium with or without Tet. The cells were fixed and stained with antiglycosome antiserum. Note the diffuse fluorescence pattern in the cells in which PEX14 RNAi was induced. (D) Digitonin fractionation confirms mislocalization of glycosomal protein upon PEX14 RNAi. Parasites were incubated with digitonin to solubilize the plasma membrane, and then glycosomes and other organelles were pelleted by centrifugation. The supernatants (S) and pellets (P) were analyzed by immunoblot analysis for the localization of cytosolic (C) and glycosomal (G) PGK, as well as mitochondrial HSP70 (M).
To verify that abrogation of TbPEX14 function compromised glycosomal protein import, populations grown in the absence of glucose were examined by immunofluorescence using the anti-glycosomal antiserum (Fig. 3C). In the uninduced population, the typical punctate glycosomal staining was observed. In the population induced for PEX14 RNAi, staining was diffuse and cytoplasmic. The loss of glycosomal compartmentation was confirmed by testing the localization of glycosomal PGK after differential digitonin solubilization of uninduced and induced cells (23). Digitonin permeabilizes the plasma membrane but not the glycosomal or inner mitochondrial membranes. A cytosolic control protein, the 45-kDa PGK isoform, was in the digitonin supernatant in both samples, whereas an organellar control protein, mitochondrial HSP70, was in the pellet fraction in both samples. Glycosomal PGK was found in the organellar fraction of control cells, but in the cytosolic fraction of cells in which PEX14 RNAi was induced (Fig. 3D). Thus, commensurate with its predicted function in protein import, reduction in TbPEX14 levels causes mislocalization of glycosomal proteins.
Interference with PEX14 Expression Kills Bloodstream Form T. brucei.
Having shown that glycosomal protein import is essential in procyclic forms, we next tested bloodstream forms. We selected stable bloodstream form transfectants containing the regulatable PEX14 RNAi construct. When PEX14 RNAi was induced, bloodstream form proliferation ceased within 2 days (Fig. 4). By 2 days, the microscopic live/dead assay showed that ≈40% of the induced parasites were dying, as compared with <8% of the uninduced parasites. Many of the parasites showed a rounded morphology and possessed one or two detached flagella (Fig. 4). Thus, PEX14 function is also essential in bloodstream stage parasites.
Fig 4.
Induction of PEX14 RNAi affects growth and viability of bloodstream forms. Growth analysis of a typical PEX14 RNAi induction in bloodstream form T. brucei. The experiment was repeated with four individual clones with similar results. The image shows an example of cellular morphology 2 days after induction of PEX14 RNAi.
Discussion
Lack of peroxisomal function, including that mediated by deficiency in PEX14, does not appear deleterious to yeast unless growth on fatty acids is required. In human peroxisomal disorders, the defects in ether-lipid biosynthesis (34), β-oxidation of very-long-chain fatty acids (35, 36), and α-oxidation of branched-chain fatty acids (37, 38) are toxic to the organism, although fibroblasts are viable in culture. In contrast, in T. brucei disruption of the peroxisomal docking factor PEX14 has a profound deleterious effect. This effect was evident both in the highly glycolytic stage that infects mammals and in the mitochondrially respiring insect stage. While this manuscript was in preparation, it was reported that RNAi-mediated disruption of PEX2, another molecule required for peroxisomal protein import, led to the death of both bloodstream and procyclic T. brucei (39).
Why does PEX14 RNAi kill trypanosomes? Metabolic interference likely contributes to cell death in the bloodstream stage, as it has been previously shown that mislocalization of the glycosomal isozyme of PGK or triose-phosphate isomerase to the cytosol in this stage is toxic (40, 41). Furthermore, hexokinase and phosphofructokinase, typically key regulators of glycolysis, are not subject to regulation in trypanosomes (42). Indeed, mathematical modeling suggests that loss of compartmentation of glycolysis would allow these enzymes to mediate toxic effects in bloodstream forms (43). We could not test whether the lethality of PEX14 RNAi is mediated by glycolytic substrates in the bloodstream stage, as the parasites cannot survive in their absence.
Until now, potential mechanisms that might explain a requirement for glycosomes in procyclic forms have not been examined. A decrease in glycolysis is unlikely to kill procyclic forms, as these parasites easily survive in the absence of glucose. Although the abundances of the glycolytic enzymes are much reduced in procyclic forms, the glycolytic capacity of procyclic forms remains relatively high (44, 45). Glucose metabolism could be toxic in procyclic forms in the absence of the regulation imposed by glycosomal compartmentation. Given that removal of simple sugars protects procyclic forms from PEX14 RNAi, we propose that mislocalization of one or more glycolytic enzymes mediates the proximal toxic effect. Because cytosolic PGK is not toxic in this stage (32), the culprit is unlikely to be PGK.
Our data support the contention that compartmentation of enzymatic pathways can provide a strong selective force for evolution and maintenance of cellular organelles. The metabolic separation resulting from physical partitioning could allow evolutionary changes in enzymes or pathways to be isolated from one another. As seen in our studies of the glycosome, subsequent loss of compartmentation could lead to catastrophic results.
Metabolic pathways contained in glycosomes have long been considered candidates for the development of antiparasitic agents. Research on glycosomal glycolytic enzymes has focused primarily on bloodstream stage T. brucei because glycolysis is their sole source of energy. Consequently, it is perhaps not surprising that disruption of glycosomal import kills bloodstream parasites, even though similar disruptions of peroxisomal protein import are not toxic to human cells (19, 20). The finding that glucose kills trypanosomes with defective glycosomal compartmentation is a bonus, as this substrate is both ubiquitously present and required in the bloodstream stage. More unexpected is our demonstration that killing of parasites based on glycosomal impairment does not require a reliance on glycolysis for energy generation. These data suggest that glycosomal compartmentation may be essential in the other pathogenic trypanosomatids, Leishmania (46) and T. cruzi (47), which are not exclusively glycolytic. Thus, the glycosomal matrix protein import machinery may provide a promising target for the development of broad-spectrum antiparasite agents.
Acknowledgments
We thank Drs. Elizabeth Wirtz and George Cross for providing the 29-13 and single-marker trypanosome lines, Dr. Steven G. Reed for anti-mitochondrial HSP70 and anti-P0, and Dr. Paul Michels for the unpublished sequence of TbPEX7. We gratefully acknowledge Drs. Pradipsinh Rathod and Kenneth Stuart for critical reading of the manuscript. This work was supported in part by National Institutes of Health Grant AI22635 (to M.P.) and a grant from the Canadian Institutes for Health Research (to A.J.).
Abbreviations
PGK, phosphoglycerate kinase
RNAi, double-stranded RNA interference
Tet, tetracycline
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Parsons M., Furuya, T., Pal, S. & Kessler, P. (2001) Mol. Biochem. Parasitol. 115, 19-28. [DOI] [PubMed] [Google Scholar]
- 2.Opperdoes F. R. & Borst, P. (1977) FEBS Lett. 80, 360-364. [DOI] [PubMed] [Google Scholar]
- 3.Heise N. & Opperdoes, F. R. (1997) Mol. Biochem. Parasitol. 89, 61-72. [DOI] [PubMed] [Google Scholar]
- 4.Zomer A. W. M., Opperdoes, F. R. & Van den Bosch, H. (1995) Biochim. Biophys. Acta 1257, 167-173. [DOI] [PubMed] [Google Scholar]
- 5.Wiemer E. A. C., Ijlst, L., Van Roy, J., Wanders, R. J. A. & Opperdoes, F. R. (1996) Mol. Biochem. Parasitol. 82, 107-111. [DOI] [PubMed] [Google Scholar]
- 6.Shih S., Hwang, H. Y., Carter, D., Stenberg, P. & Ullman, B. (1998) J. Biol. Chem. 273, 1534-1541. [DOI] [PubMed] [Google Scholar]
- 7.Clayton C. E. & Michels, P. (1996) Parasitol. Today 12, 465-471. [DOI] [PubMed] [Google Scholar]
- 8.Michels P. A. (1988) Biol. Cell 64, 157-164. [DOI] [PubMed] [Google Scholar]
- 9.Verlinde C. L., Hannaert, V., Blonski, C., Willson, M., Perie, J. J., Fothergill-Gilmore, L. A., Opperdoes, F. R., Gelb, M. H., Hol, W. G. & Michels, P. A. (2001) Drug Resist. Updat. 4, 50-65. [DOI] [PubMed] [Google Scholar]
- 10.Jardim A., Liu, W., Zheleznova, E. & Ullman, B. (2000) J. Biol. Chem. 275, 13637-13644. [DOI] [PubMed] [Google Scholar]
- 11.de Walque S., Kiel, J. A., Veenhuis, M., Opperdoes, F. R. & Michels, P. A. (1999) Mol. Biochem. Parasitol. 104, 106-119. [DOI] [PubMed] [Google Scholar]
- 12.Flaspohler J. A., Rickoll, W. L., Beverley, S. M. & Parsons, M. (1997) Mol. Cell. Biol. 17, 1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz P., Maier, A. G., Baumgart, E., Erdmann, R. & Clayton, C. (1998) EMBO J. 17, 3542-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Roermund C. W., Tabak, H. F., Van den Berg, M., Wanders, R. J. & Hettema, E. H. (2000) J. Cell Biol. 150, 489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X. & Gould, S. J. (2002) J. Cell Biol. 156, 643-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaspohler J. A., Lemley, K. & Parsons, M. (1999) Mol. Biochem. Parasitol. 99, 117-128. [DOI] [PubMed] [Google Scholar]
- 17.Fransen M., Terlecky, S. R. & Subramani, S. (1998) Proc. Natl. Acad. Sci. USA 95, 8087-8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson M. A., Snyder, W. B., Cereghino, J. L., Veenhuis, M., Subramani, S. & Cregg, J. M. (2001) Yeast 18, 621-641. [DOI] [PubMed] [Google Scholar]
- 19.Komori M., Rasmussen, S. W., Kiel, J. A. K. W., Baerends, R. J. S., Cregg, J. M., Van der Klei, I. J. & Veenhuis, M. (1997) EMBO J. 16, 44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albertini M., Rehling, P., Erdmann, R., Girzalsky, W., Kiel, J. A. K. W., Veenhuis, M. & Kunau, W. H. (1997) Cell 89, 83-92. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Morris, J. C., Drew, M. E. & Englund, P. T. (2000) J. Biol. Chem. 275, 40174-40179. [DOI] [PubMed] [Google Scholar]
- 22.Parsons M. & Nielsen, B. (1990) Exp. Parasitol. 70, 276-285. [DOI] [PubMed] [Google Scholar]
- 23.Sommer J. M., Cheng, Q.-L., Keller, G.-A. & Wang, C. C. (1992) Mol. Biol. Cell 3, 749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiki Y., Hubbard, A. L., Fowler, S. & Lazarow, P. B. (1982) J. Cell Biol. 61, 97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker H. L., Hill, T., Alexander, K., Murphy, N. B., Fish, W. R. & Parsons, M. (1995) Mol. Biochem. Parasitol. 69, 269-279. [DOI] [PubMed] [Google Scholar]
- 26.Skeiky Y. A. W., Benson, D. R., Guderian, J. A., Sleath, P. R., Parsons, M. & Reed, S. G. (1993) J. Immunol. 151, 1-12. [PubMed] [Google Scholar]
- 27.Wirtz E., Leal, S., Ochatt, C. & Cross, G. A. (1999) Mol. Biochem. Parasitol. 99, 89-101. [DOI] [PubMed] [Google Scholar]
- 28.Brun R. & Schononberger, M. (1979) Acta Trop. 36, 289-292. [PubMed] [Google Scholar]
- 29.Carruthers V. B. & Cross, G. A. M. (1992) Proc. Natl. Acad. Sci. USA 89, 8818-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnaufer A., Panigrahi, A. K., Panicucci, B., Igo, R. P. J., Wirtz, E., Salavati, R. & Stuart, K. (2001) Science 291, 2159-2162. [DOI] [PubMed] [Google Scholar]
- 31.Hirumi H. & Hirumi, K. (1989) J. Parasitol. 75, 985-989. [PubMed] [Google Scholar]
- 32.Osinga K. A., Swinkels, B. W., Gibson, W. C., Borst, P., Veeneman, G. H., Van Boom, J. H., Michels, P. A. M. & Opperdoes, F. R. (1985) EMBO J. 4, 3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu N., Itoh, R., Hirono, Y., Otera, H., Ghaedi, K., Tateishi, K., Tamura, S., Okumoto, K., Harano, T., Mukai, S. & Fujiki, Y. (1999) J. Biol. Chem. 274, 12593-12604. [DOI] [PubMed] [Google Scholar]
- 34.Datta N. S., Wilson, G. N. & Hajra, A. K. (1984) N. Engl. J. Med. 311, 1080-1083. [DOI] [PubMed] [Google Scholar]
- 35.Moser H. W. & Moser, A. B. (1996) Ann. N.Y. Acad. Sci. 804, 427-441. [DOI] [PubMed] [Google Scholar]
- 36.Moser A. E., Singh, I., Brown, F. R., Solish, G. I., Kelley, R. I., Benke, P. J. & Moser, H. W. (1984) N. Engl. J. Med. 310, 1141-1146. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein R. (1999) J. Clin. Apheresis 14, 181-184. [DOI] [PubMed] [Google Scholar]
- 38.Mukherji M., Chien, W., Kershaw, N. J., Clifton, I. J., Schofield, C. J., Wierzbicki, A. S. & Lloyd, M. D. (2001) Hum. Mol. Genet. 10, 1971-1982. [DOI] [PubMed] [Google Scholar]
- 39.Guerra-Giraldez C., Quijada, L. & Clayton, C. E. (2002) J. Cell Sci. 115, 2651-2658. [DOI] [PubMed] [Google Scholar]
- 40.Blattner J., Helfert, S., Michels, P. & Clayton, C. (1998) Proc. Natl. Acad. Sci. USA 95, 11596-11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helfert S., Estevez, A. M., Bakker, B., Michels, P. & Clayton, C. (2001) Biochem. J. 357, 117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nwagwu M. & Opperdoes, F. R. (1982) Acta Trop. 39, 61-72. [PubMed] [Google Scholar]
- 43.Bakker B. M., Mensonides, F. I., Teusink, B., van Hoek, P., Michels, P. A. & Westerhoff, H. V. (2000) Proc. Natl. Acad. Sci. USA 97, 2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryley J. F. (1962) Biochem. J. 85, 211-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ter Kuile B. H. (1997) J. Bacteriol. 179, 4699-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart D. T., Vickerman, K. & Coombs, G. H. (1981) Mol. Biochem. Parasitol. 4, 39-51. [DOI] [PubMed] [Google Scholar]
- 47.Urbina J. A., Machin, I. & Jurado, L. (1993) Biol. Res. 26, 81-88. [PubMed] [Google Scholar]