Abstract
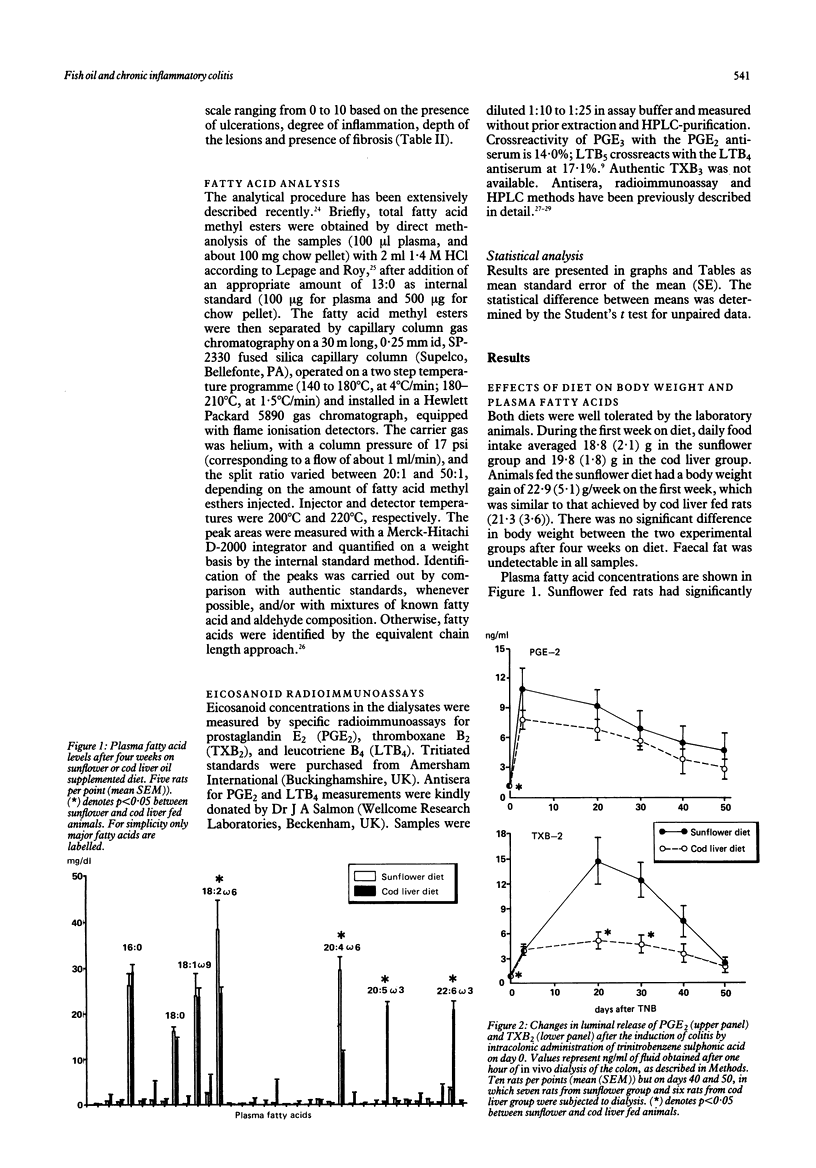
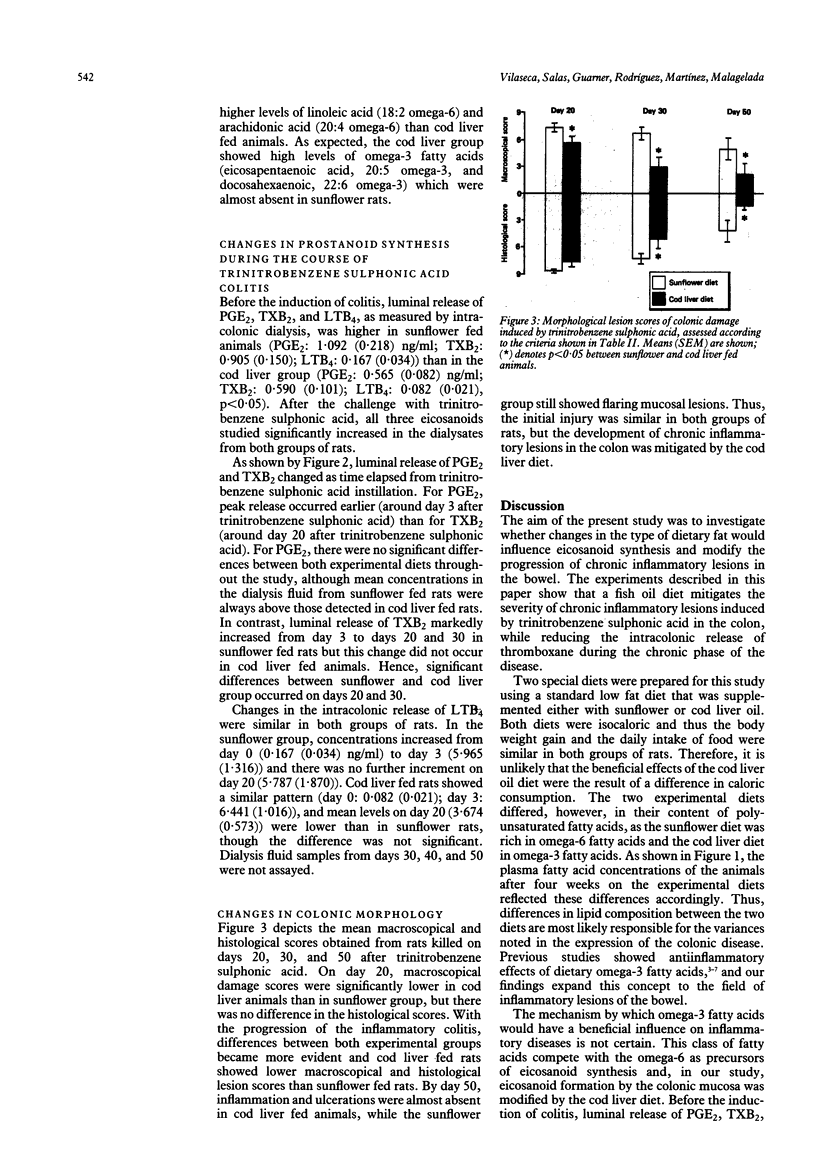
Eicosanoids are modulators of defensive and inflammatory processes in the gut mucosa, and may be involved in the pathogenesis of chronic inflammatory lesions of the bowel. As omega-3 fatty acids compete with the omega-6 as precursors of eicosanoid synthesis, we compared the effects of dietary supplementation with either sunflower (source of omega-6) or cod liver (source of omega-3) oil on the development of chronic granulomatous lesions in the rat colon. After four weeks on the supplemented diets, plasma omega-6 fatty acid content was significantly higher in the sunflower group, while omega-3 fatty acids predominated in the cod liver group. Inflammatory colitis was then induced by intracolonic administration of trinitrobenzene sulphonic acid. Luminal eicosanoid release, as measured by radioimmunoassay of intracolonic dialysis fluid, increased significantly after the challenge in both groups. Generation of prostaglandin E2 (PGE2) and leucotriene B4 (LTB4) peaked by day 3 and thereafter declined; thromboxane B2 (TXB2), instead, continued to increase from day 3 to 20 in sunflower fed rats, whereas this change was blunted in cod liver animals. The rats were killed 20, 30, or 50 days after the induction of colitis, and the colonic lesions were scored macroscopically (adhesions to surrounding tissues, strictures, ulcerations, and wall thickness) and histologically (ulceration, inflammation, depth of the lesions, and fibrosis). In cod liver animals, the damage score was markedly reduced by day 30, and inflammation and ulceration were almost absent by day 50. In conclusion, a fish oil diet prevents the increase in thromboxane in the chronic state of inflammation and shortens the course of the colonic disease by diminishing both the severity of the lesions and their progression to chronicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist D. A., Duenes J. A., Madson T. H., Romero J. C., Dozois R. R., Malagelada J. R. Prostaglandin generation from gastroduodenal mucosa: regional and species differences. Prostaglandins. 1982 Jul;24(1):115–125. doi: 10.1016/0090-6980(82)90183-6. [DOI] [PubMed] [Google Scholar]
- Bittiner S. B., Tucker W. F., Cartwright I., Bleehen S. S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988 Feb 20;1(8582):378–380. doi: 10.1016/s0140-6736(88)91181-6. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Hawkey C. J., Whittle B. J. Biosynthesis of lipoxygenase and cyclo-oxygenase products from [14C]-arachidonic acid by human colonic mucosa. Gut. 1983 Dec;24(12):1176–1182. doi: 10.1136/gut.24.12.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Wallace J. L., Morris G. P., Whittle B. J. The effect of anti-inflammatory drugs on eicosanoid formation in a chronic model of inflammatory bowel disease in the rat. Br J Pharmacol. 1988 May;94(1):65–72. doi: 10.1111/j.1476-5381.1988.tb11500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Kelley V. E., Georgilis K., Lonnemann G., van der Meer J. W., Cannon J. G., Rogers T. S., Klempner M. S., Weber P. C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- Guarner C., Colina I., Guarner F., Corzo J., Prieto J., Vilardell F. Renal prostaglandins in cirrhosis of the liver. Clin Sci (Lond) 1986 May;70(5):477–484. doi: 10.1042/cs0700477. [DOI] [PubMed] [Google Scholar]
- Hawkey C. J., Karmeli F., Rachmilewitz D. Imbalance of prostacyclin and thromboxane synthesis in Crohn's disease. Gut. 1983 Oct;24(10):881–885. doi: 10.1136/gut.24.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G. A., Moncada S., Salmon J. A., Seager K. The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol. 1983 Aug;79(4):863–868. doi: 10.1111/j.1476-5381.1983.tb10530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. R. GLC identification techniques for long-chain unsaturated fatty acids. J Chromatogr Sci. 1975 Oct;13(10):491–497. doi: 10.1093/chromsci/13.10.491. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kremer J. M., Jubiz W., Michalek A., Rynes R. I., Bartholomew L. E., Bigaouette J., Timchalk M., Beeler D., Lininger L. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987 Apr;106(4):497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- Lauritsen K., Hansen J., Bytzer P., Bukhave K., Rask-Madsen J. Effects of sulphasalazine and disodium azodisalicylate on colonic PGE2 concentrations determined by equilibrium in vivo dialysis of faeces in patients with ulcerative colitis and healthy controls. Gut. 1984 Nov;25(11):1271–1278. doi: 10.1136/gut.25.11.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986 Oct;91(4):837–844. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. In vivo effects of orally administered prednisolone on prostaglandin and leucotriene production in ulcerative colitis. Gut. 1987 Sep;28(9):1095–1099. doi: 10.1136/gut.28.9.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen K., Laursen L. S., Bukhave K., Rask-Madsen J. In vivo profiles of eicosanoids in ulcerative colitis, Crohn's colitis, and Clostridium difficile colitis. Gastroenterology. 1988 Jul;95(1):11–17. doi: 10.1016/0016-5085(88)90284-3. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Lepage G., Roy C. C. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986 Jan;27(1):114–120. [PubMed] [Google Scholar]
- Ligumsky M., Karmeli F., Sharon P., Zor U., Cohen F., Rachmilewitz D. Enhanced thromboxane A2 and prostacyclin production by cultured rectal mucosa in ulcerative colitis and its inhibition by steroids and sulfasalazine. Gastroenterology. 1981 Sep;81(3):444–449. [PubMed] [Google Scholar]
- Martinez M. Polyunsaturated fatty acid changes suggesting a new enzymatic defect in Zellweger syndrome. Lipids. 1989 Apr;24(4):261–265. doi: 10.1007/BF02535160. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Mehta J. L., Lopez L. M., Lawson D., Wargovich T. J., Williams L. L. Dietary supplementation with omega-3 polyunsaturated fatty acids in patients with stable coronary heart disease. Effects on indices of platelet and neutrophil function and exercise performance. Am J Med. 1988 Jan;84(1):45–52. doi: 10.1016/0002-9343(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc Natl Acad Sci U S A. 1979 Feb;76(2):944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskar B. M., Dreyling K. W., Peskar B. A., May B., Goebell H. Enhanced formation of sulfidopeptide-leukotrienes in ulcerative colitis and Crohn's disease: inhibition by sulfasalazine and 5-aminosalicylic acid. Agents Actions. 1986 Jun;18(3-4):381–383. doi: 10.1007/BF01965001. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., Prickett J. D., Makoul G. T., Steinberg A. D., Colvin R. B. Dietary fish oil reduces progression of established renal disease in (NZB x NZW)F1 mice and delays renal disease in BXSB and MRL/1 strains. Arthritis Rheum. 1986 Apr;29(4):539–546. doi: 10.1002/art.1780290412. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Simmons P. M., Palmer R. M. A radioimmunoassay for leukotriene B4. Prostaglandins. 1982 Aug;24(2):225–235. doi: 10.1016/0090-6980(82)90148-4. [DOI] [PubMed] [Google Scholar]
- Sharon P., Ligumsky M., Rachmilewitz D., Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978 Oct;75(4):638–640. [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984 Mar;86(3):453–460. [PubMed] [Google Scholar]
- Spagnuolo P. J., Ellner J. J., Hassid A., Dunn M. J. Thromboxane A2 mediates augmented polymorphonuclear leukocyte adhesiveness. J Clin Invest. 1980 Sep;66(3):406–414. doi: 10.1172/JCI109870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano T., Salmon J. A., Higgs G. A., Moncada S. Eicosapentaenoic acid as a modulator of inflammation. Effect on prostaglandin and leukotriene synthesis. Biochem Pharmacol. 1986 Mar 1;35(5):779–785. doi: 10.1016/0006-2952(86)90246-7. [DOI] [PubMed] [Google Scholar]
- Terano T., Salmon J. A., Moncada S. Biosynthesis and biological activity of leukotriene B5. Prostaglandins. 1984 Feb;27(2):217–232. doi: 10.1016/0090-6980(84)90075-3. [DOI] [PubMed] [Google Scholar]
- Vilaseca J., Salas A., Guarner F., Rodriguez R., Malagelada J. R. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990 Feb;98(2):269–277. doi: 10.1016/0016-5085(90)90814-h. [DOI] [PubMed] [Google Scholar]


