Abstract
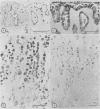
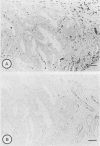
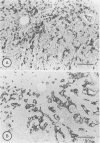
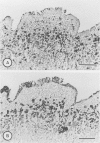
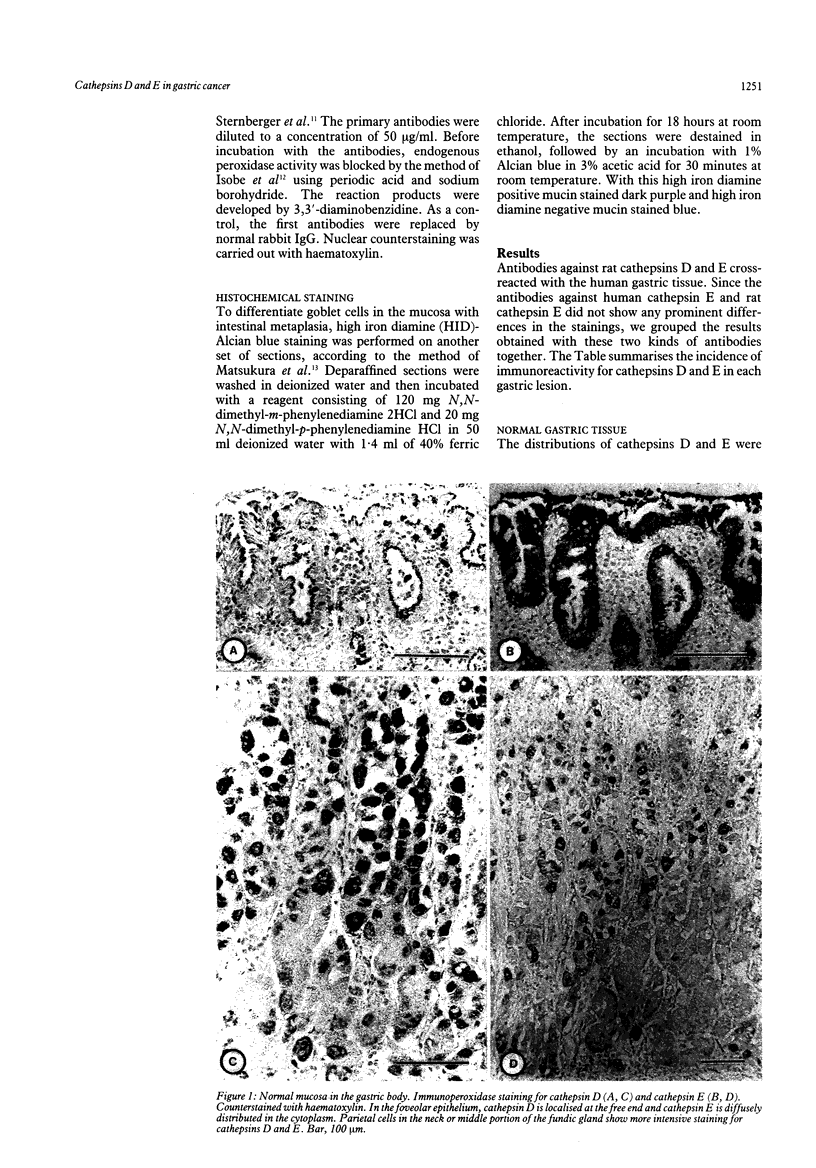
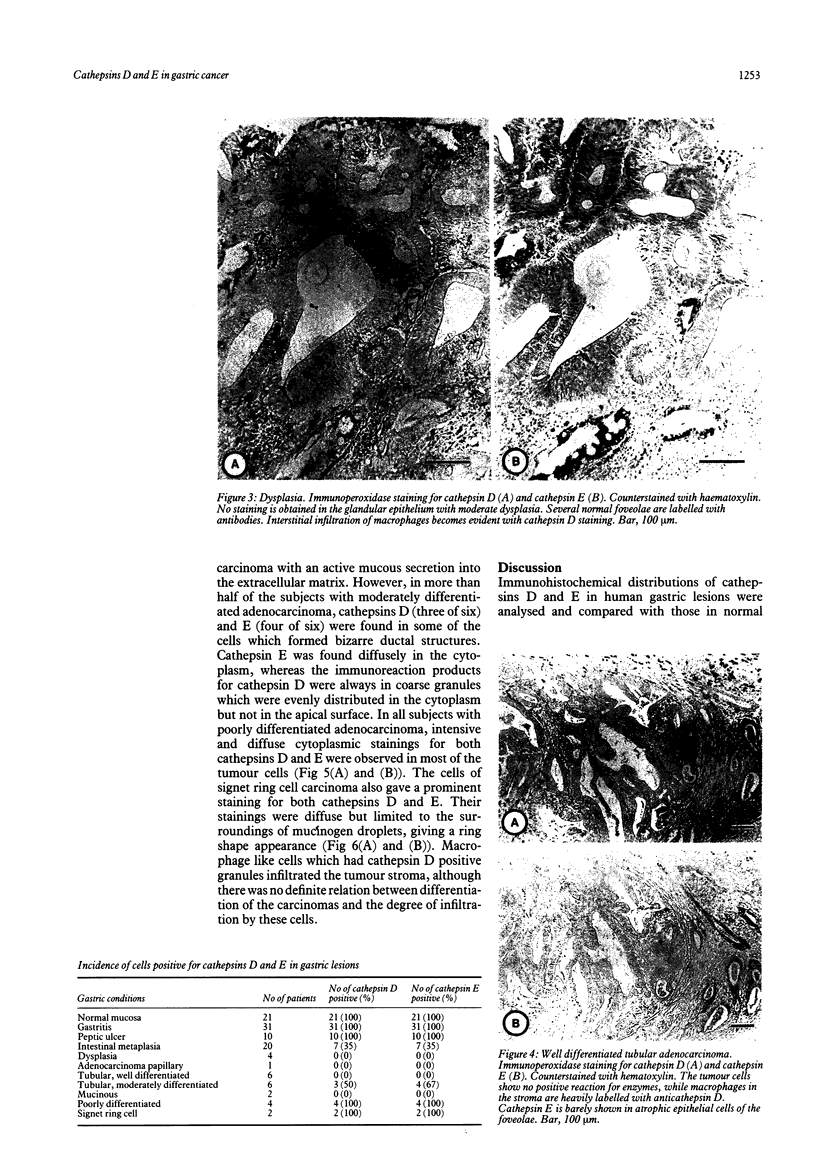
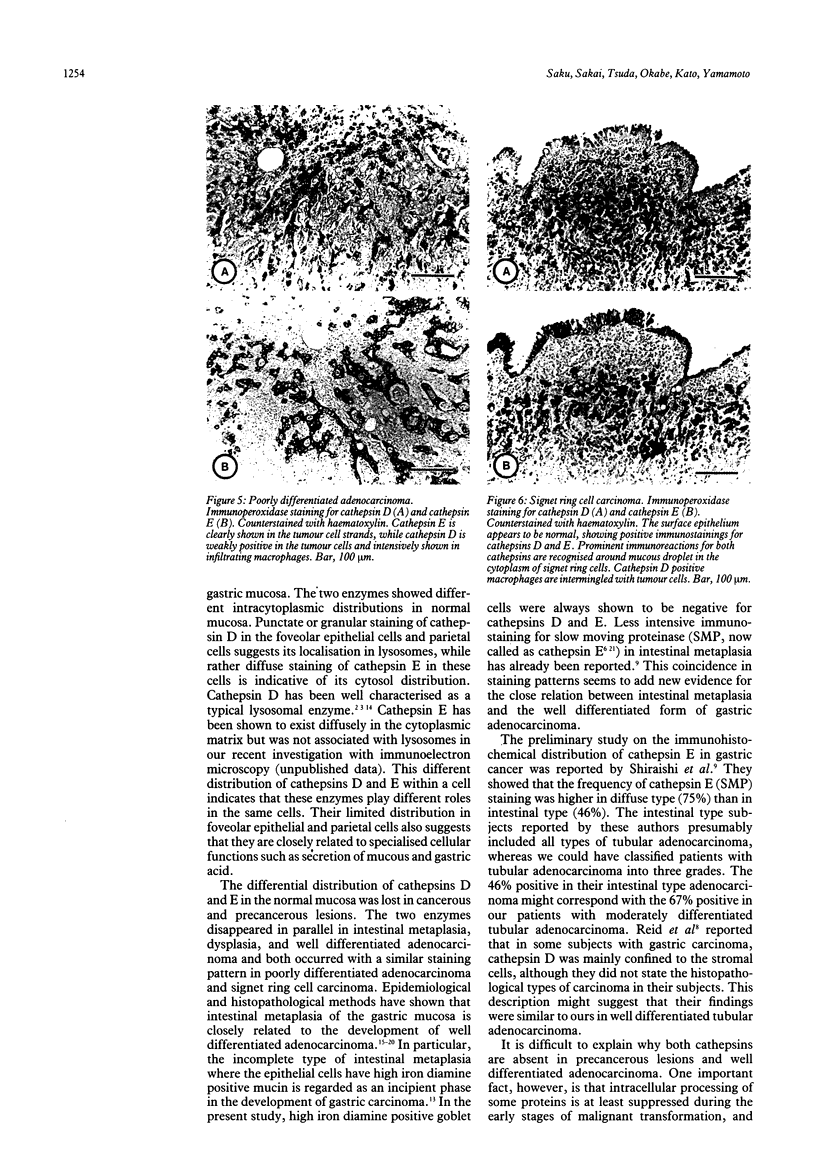
Immunohistochemical distributions of cathepsins D and E were determined in normal mucosa, metaplastic, dysplastic, and cancerous lesions of the human stomach. Cathepsins D and E were localised in the foveolar epithelium and parietal cells of the normal gastric mucosa, but their intracytoplasmic distributions were different - cathepsin E distribution was even and diffuse in the cytoplasm while cathepsin D was found in coarse intracytoplasmic granules. Chronic inflammation and ulcer did not influence the distribution of these enzymes. No positive staining was obtained in the incomplete type of intestinal metaplasia, dysplasia, and well differentiated adenocarcinoma. Tumour cells of signet ring cell carcinoma and poorly differentiated adenocarcinoma cells, however, gave strong and diffuse stainings for cathepsins D and E in the cytoplasm. The results suggest that the distribution of cathepsins D and E is related to each specialised function of the foveolar epithelium and the parietal cells, and that their disappearance is associated with development of well differentiated adenocarcinoma from intestinal metaplasia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuello C., Correa P., Haenszel W., Gordillo G., Brown C., Archer M., Tannenbaum S. Gastric cancer in Colombia. I. Cancer risk and suspect environmental agents. J Natl Cancer Inst. 1976 Nov;57(5):1015–1020. doi: 10.1093/jnci/57.5.1015. [DOI] [PubMed] [Google Scholar]
- Iida F., Kusama J. Gastric carcinoma and intestinal metaplasia. Significance of types of intestinal metaplasia upon development of gastric carcinoma. Cancer. 1982 Dec 15;50(12):2854–2858. doi: 10.1002/1097-0142(19821215)50:12<2854::aid-cncr2820501227>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Imai T., Kubo T., Watanabe H. Chronic gastritis in Japanese with reference to high incidence of gastric carcinoma. J Natl Cancer Inst. 1971 Jul;47(1):179–195. [PubMed] [Google Scholar]
- Jupp R. A., Richards A. D., Kay J., Dunn B. M., Wyckoff J. B., Samloff I. M., Yamamoto K. Identification of the aspartic proteinases from human erythrocyte membranes and gastric mucosa (slow-moving proteinase) as catalytically equivalent to cathepsin E. Biochem J. 1988 Sep 15;254(3):895–898. doi: 10.1042/bj2540895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S. H., Bezuidenhout D. J. Enzymatic analysis of gastric microbiopsy specimens. An aid in the differential diagnosis between peptic ulcers and gastric carcinoma? Cancer. 1983 May 1;51(9):1653–1655. doi: 10.1002/1097-0142(19830501)51:9<1653::aid-cncr2820510917>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Matsukura N., Suzuki K., Kawachi T., Aoyagi M., Sugimura T., Kitaoka H., Numajiri H., Shirota A., Itabashi M., Hirota T. Distribution of marker enzymes and mucin in intestinal metaplasia in human stomach and relation to complete and incomplete types of intestinal metaplasia to minute gastric carcinomas. J Natl Cancer Inst. 1980 Aug;65(2):231–240. [PubMed] [Google Scholar]
- Miki K., Oda T., Suzuki H., Iino S., Niwa H. Alkaline phosphatase isoenzymes in intestinal metaplasia and carcinoma of the stomach. Cancer Res. 1976 Nov;36(11 Pt 2):4266–4268. [PubMed] [Google Scholar]
- Ming S. C., Goldman H., Freiman D. G. Intestinal metaplasia and histogenesis of carcinoma in human stomach. Light and electron microscopic study. Cancer. 1967 Sep;20(9):1418–1429. doi: 10.1002/1097-0142(196709)20:9<1418::aid-cncr2820200908>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Muto N., Yamamoto M., Tani S., Yonezawa S. Characteristic distribution of cathepsin E which immunologically cross-reacts with the 86-kDa acid proteinase from rat gastric mucosa. J Biochem. 1988 Apr;103(4):629–632. doi: 10.1093/oxfordjournals.jbchem.a122318. [DOI] [PubMed] [Google Scholar]
- Puizdar V., Lapresle C., Turk V. Evidence for the presence of large amounts of cathepsin E in rat spleen. FEBS Lett. 1985 Jun 17;185(2):236–238. doi: 10.1016/0014-5793(85)80913-3. [DOI] [PubMed] [Google Scholar]
- Reid W. A., Valler M. J., Kay J. Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol. 1986 Dec;39(12):1323–1330. doi: 10.1136/jcp.39.12.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Saku T., Kato Y., Yamamoto K. Quantitation and immunohistochemical localization of cathepsins E and D in rat tissues and blood cells. Biochim Biophys Acta. 1989 May 31;991(2):367–375. doi: 10.1016/0304-4165(89)90130-x. [DOI] [PubMed] [Google Scholar]
- Samloff I. M., Taggart R. T., Shiraishi T., Branch T., Reid W. A., Heath R., Lewis R. W., Valler M. J., Kay J. Slow moving proteinase. Isolation, characterization, and immunohistochemical localization in gastric mucosa. Gastroenterology. 1987 Jul;93(1):77–84. [PubMed] [Google Scholar]
- Shiraishi T., Samloff I. M., Taggart R. T., Stemmermann G. N. Slow moving proteinase in gastric cancer and its relationship to pepsinogens I and II. An immunohistochemical study. Dig Dis Sci. 1988 Nov;33(11):1466–1472. doi: 10.1007/BF01537004. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kamata O., Katsuda N., Kato K. Immunochemical difference between cathepsin D and cathepsin E-like enzyme from rat spleen. J Biochem. 1980 Feb;87(2):511–516. doi: 10.1093/oxfordjournals.jbchem.a132772. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Katsuda N., Himeno M., Kato K. Cathepsin D of rat spleen. Affinity purification and properties of two types of cathepsin D. Eur J Biochem. 1979 Apr;95(3):459–467. doi: 10.1111/j.1432-1033.1979.tb12985.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Katsuda N., Kato K. Affinity purification and properties of cathepsin-E-like acid proteinase from rat spleen. Eur J Biochem. 1978 Dec;92(2):499–508. doi: 10.1111/j.1432-1033.1978.tb12772.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ueno E., Uemura H., Kato Y. Biochemical and immunochemical similarity between erythrocyte membrane aspartic proteinase and cathepsin E. Biochem Biophys Res Commun. 1987 Oct 14;148(1):267–272. doi: 10.1016/0006-291x(87)91105-3. [DOI] [PubMed] [Google Scholar]
- Yokota S., Tsuji H., Kato K. Immunocytochemical localization of cathepsin D in lysosomes of cortical collecting tubule cells of the rat kidney. J Histochem Cytochem. 1985 Mar;33(3):191–200. doi: 10.1177/33.3.2579120. [DOI] [PubMed] [Google Scholar]