Abstract
Caloric restriction extends life span in a variety of species, highlighting the importance of energy balance in aging. A new longevity gene, Indy (for I'm not dead yet), which doubles the average life span of flies without a loss of fertility or physical activity, was postulated to extend life by affecting intermediary metabolism. We report that functional studies in Xenopus oocytes show INDY is a metabolite transporter that mediates the high-affinity, disulfonic stilbene-sensitive flux of dicarboxylates and citrate across the plasma membrane by a mechanism that is not coupled to Na+, K+, or Cl−. Immunocytochemical studies localize INDY to the plasma membrane with most prominent expression in adult fat body, oenocytes, and the basolateral region of midgut cells and show that life-extending mutations in Indy reduce INDY expression. We conclude that INDY functions as a novel sodium-independent mechanism for transporting Krebs and citric acid cycle intermediates through the epithelium of the gut and across the plasma membranes of organs involved in intermediary metabolism and storage. The life-extending effect of mutations in Indy is likely caused by an alteration in energy balance caused by a decrease in INDY transport function.
Caloric restriction is the only known means of extending life span in mammals (1). Although the mechanism by which caloric restriction extends life span is not understood, it is likely to include alterations in energy utilization. Caloric restriction has been shown to increase life span in a variety of other species including fruit flies, supporting the idea that energy balance is a critical element in the aging of many species (1, 2). A new class of longevity gene, Indy (for I'm not dead yet), was identified in the fly and postulated to extend life span through its effect on intermediary metabolism (3). Mutations in the Indy gene result in a near doubling of the average life span of adult flies without a loss of fertility or physical activity (3). The function of the INDY protein was not known, but its closest homology (34% identity) to mammalian sodium-dicarboxylate cotransporters (4–12) and the tissue distribution of its transcriptional activity (fat body, oenocytes, and midgut; ref. 3) suggested that mutations in this gene may be reducing transport of important nutrients in tissues critical for intermediary metabolism. Direct knowledge of the function of the INDY protein and its subcellular distribution is essential to our understanding of how life span can be so dramatically increased by a single gene mutation.
Materials and Methods
Cloning of Indy for Expression Studies.
The coding sequence was amplified by PCR using primers designed to incorporate upstream and downstream BamHI and XbaI sites, respectively (5′ primer: ATAAGAAGGATCCACCATGGAAATTGAAATTGGCGAACAACC-oh; 3′ primer: CGGTCTAGACTAGTGCGTCTTGTTTCCCAGTG-oh). The BamHI and XbaI sites then were used to subclone the PCR product into the Xenopus expression plasmid pGH19 between the 5′ and 3′ UTRs of the Xenopus β-globin gene (13). Indy sequence was confirmed by sequencing on an ABI automated sequencer (University of Connecticut Health Center Core Facility).
pGH19 plasmid DNA was linearized by XhoI digestion, and cRNA was transcribed by using T7 RNA polymerase (mMESSAGE mMACHINE, Ambion, Austin, TX). Precipitated cRNA was dissolved in sterile H2O, and yield and quality were assessed by spectroscopy and agarose gel electrophoresis.
Functional Expression in Xenopus Oocytes.
Studies were performed by using methods similar to those described (14). In brief, oocytes were isolated from Xenopus laevisfrogs by partial ovariectomy and washed for 20 min in Ca-free hypotonic medium (85 mM NaCl/2 mM KCl/1 mM MgCl2/5 mM Hepes titrated with Tris base to pH 7.5). Oocytes then were defolliculated by treatment with 2 mg/ml collagenase (Worthington type I or Sigma type I) in Ca-free hypotonic solution for 45–90 min with gentle agitation at room temperature. After this incubation, oocytes were washed three times in Ca-free hypotonic media, then washed three times in isotonic solution (96 mM NaCl/2.0 mM KCl/1.8 mM CaCl2/1.0 mM MgCl2/5 mM Hepes, pH 7.5). Stage V–VI oocytes then were selected and maintained at 18°C in the same isotonic solution supplemented with 50 mg/ml gentamicin and 2.5 mM sodium pyruvate. On the day of their isolation, oocytes were loaded by use of a pneumatic injector with 50 nl of sterile H2O or 50 nl of a cRNA solution containing 25 ng of Indy cRNA. In one set of experiments, oocytes also were injected with 25 ng of cRNA transcribed from a cDNA encoding flounder NaDC-3 (15). The injected oocytes were incubated at 18°C for 48 h to allow for expression of the protein. For uptake experiments, oocytes were washed twice at room temperature in 1 ml transport buffer (100 mM NaCl/5 mM Hepes, pH 7.5) before incubation in 500 μl of the same solution containing [14C]succinate (18 μM) or [14C]citrate (9 μM). After a 30-min incubation, external isotope was removed by washing the oocytes three times with 1 ml of ice-cold buffer. Radioisotope content of each individual oocyte was measured by scintillation spectroscopy after solubilization in 0.2 ml of 10% (vol/vol) SDS and addition of 3 ml of scintillation fluid (Opti-Fluor, Packard). Results shown in the bar graphs represent means ± SE for 7–10 oocytes. For the experiment in which succinate uptake was measured as a function of succinate concentration (see Fig. 3), net INDY-mediated flux was calculated as the difference between uptake measured in Indy cRNA-injected oocytes and that measured in water-injected oocytes.
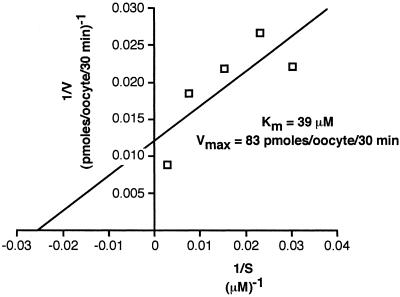
Fig 3.
INDY is a relatively high-affinity succinate transporter. Lineweaver–Burk plot indicates relatively high affinity (Km = 39 μM) for succinate transport mediated by INDY.
Preparation of Antibodies.
Independent antibodies were raised to two different peptides of INDY: EPQYQIVGGNKKNNEDE and RPKSKEAQEVQRGREGADVA. Multiple antigenic peptides (MAPs) were synthesized for each peptide (16). MAPs were mixed with Freund's adjuvant and injected into two New Zealand White rabbits per peptide. Each of the four rabbits was boosted on days 14, 42, and 56 after the primary injection. A control prebleed was taken before injection, and then subsequent bleeds were taken at days 28, 56, and 72 after primary injection and tested in an ELISA assay using the originally injected peptides (Research Genetics, Huntsville, AL). The prebleed antisera showed no specific immunostaining on adult Drosophila tissue sections.
Tissue Preparation and Immunostaining.
Adult flies 10 days old were fixed in 2% (vol/vol) paraformaldehyde and embedded in paraffin, and 5-μm sections were made. Immunostaining was done by using either of the two rabbit anti-INDY peptide antibodies at 1:5,000 and a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase at 1:200 and developed as described in Vectastain Elite ABC kit. Both anti-peptide antibodies showed the same specificity. The preimmune rabbit antibody from each showed no specific staining on control tissue sections at 1:500–1:5,000.
Results
INDY Transports Krebs Cycle Intermediates.
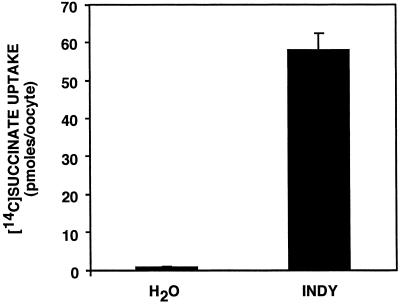
To characterize its physiological function, we heterologously expressed Indy by cRNA injection in Xenopus oocytes. Because of the homology between INDY and dicarboxylate transporters, we used [14C]succinate as a test substrate. As shown in Fig. 1, [14C]succinate uptake was increased >50-fold in oocytes injected with Indy cRNA as compared with water-injected controls. These results demonstrate that, like the mammalian sodium-dicarboxylate cotransporters, INDY can function as a succinate transporter.
Fig 1.
INDY is a transporter. A >50-fold increase in [14C]succinate transport was seen in Xenopus oocytes injected with Indy cRNA, as compared with water-injected controls.
Selectivity of the INDY Transporter.
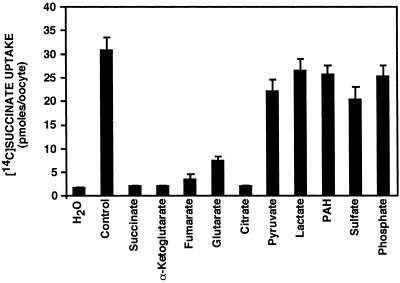
The mammalian sodium-dicarboxylate cotransporters show a selectivity in the compounds that they transport. To evaluate the anion specificity of INDY, we compared the effects of the unlabeled dicarboxylates succinate (α-ketoglutarate, fumarate, and glutarate), the tricarboxylate citrate, and the monocarboxylates pyruvate and lactate added to the external solution (10 mM) as inhibitors of uptake of 18 μM [14C]succinate. The inhibition studies (Fig. 2) suggest that, similarly to mammalian sodium-dicarboxylate cotransporters (17), INDY has affinity for succinate, α-ketoglutarate, fumarate, glutarate, and citrate, but not for pyruvate or lactate.
Fig 2.
INDY's transport selectivity is similar to mammalian sodium-dicarboxylate cotransporters. Effects of 10 mM of each indicated anion (as sodium salts) on INDY-mediated [14C]succinate uptake were assayed by iso-osmotic replacement of NaCl in the media. [14C]Succinate transport was strongly inhibited by succinate (as expected for a saturable transport process), citrate, α-ketoglutarate, glutarate, and fumarate but not by pyruvate, lactate, PAH, sulfate, or phosphate.
In the mammalian kidney, dicarboxylate transporters are involved in the excretion of anionic xenobiotics, drugs, and environmental toxicants (18). The prototypic organic anion transport mechanism mediating xenobiotic secretion is the p-aminohippurate (PAH)/dicarboxylate exchanger (19–22). As shown in Fig. 2, PAH did not significantly inhibit INDY-mediated [14C]succinate uptake, indicating that INDY is not functionally similar to the mammalian renal organic anion transporter.
Dicarboxylate carriers in the mitochondrial inner membrane mediate the transport of dicarboxylates such as malate and succinate in exchange for phosphate and sulfate (23–25). Therefore, we examined the affinity of phosphate and sulfate for INDY. As illustrated in Fig. 2, sulfate and phosphate caused minimal inhibition of INDY-mediated [14C]succinate transport.
INDY Is a High-Affinity Succinate Transporter.
INDY-mediated succinate influx was measured as a function of succinate concentration, and the resulting Lineweaver–Burk plot is illustrated in Fig. 3. The Km for succinate was ≈40 μM, a value similar to that of high-affinity mammalian sodium-dicarboxylate cotransporters (17).
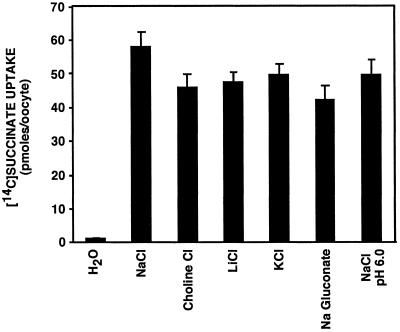
INDY Transport Is Not Cation Dependent.
Surprisingly, although the anion specificity of INDY was found to be similar to mammalian sodium-dicarboxylate transporters, succinate transport mediated by INDY is not cation dependent. As shown in Fig. 4, the uptake of succinate was not altered by the replacement of sodium with choline, lithium, or potassium. To verify that the lack of sodium-dependence of INDY-mediated transport was not an experimental artifact, we confirmed that succinate uptake into NaDC-3 cRNA-injected oocytes was 40-fold higher in the presence of sodium compared with choline (not shown). INDY also does not use protons as an alternative mechanism to mediate secondary active uptake of dicarboxylates across the cell membrane. Succinate uptake was unaffected by the imposition of an outside-acid pH gradient (Fig. 4). Thus, despite the similarity in specificity of anion transport between the mammalian sodium-dicarboxylate cotransporters and INDY, the results showing a lack of cation- or pH-dependence argue that INDY does not function as a cotransporter. The possibility that INDY transports succinate in exchange with chloride anions is also unlikely, as imposing an outward chloride gradient by replacing chloride in the external medium by gluconate did not affect the rate of INDY-mediated succinate uptake (Fig. 4).
Fig 4.
INDY is not a cotransporter. INDY-mediated transport of succinate is not coupled to Na+, K+, or H+ or driven by an exchange of Cl−. The replacement of NaCl by KCl, choline Cl, LiCl, KCl, or Na gluconate or imposition of an outside-acid pH gradient (pH 6.0) had no effect on the uptake of succinate by the INDY-expressing oocytes.
INDY's Anion Transport-Inhibitor Profile Is Unique.
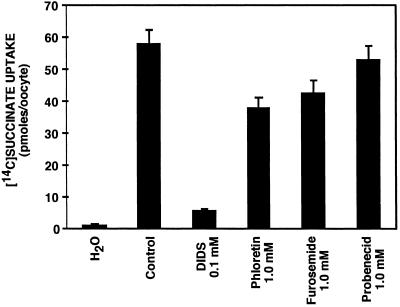
As an additional approach to compare the properties of INDY with previously described dicarboxylate transporters, we tested the effects of several anion transport inhibitors. As indicated in Fig. 5, INDY-mediated [14C]succinate transport was almost completely inhibited by 0.1 mM DIDS. Modest but significant inhibition was observed with 1 mM phloretin or furosemide. Probenecid (1 mM) had no significant inhibitory effect. This pattern of inhibitor sensitivity differs from that described for other plasma membrane dicarboxylate transporters. For example, mammalian sodium-dicarboxylate cotransporters are insensitive to inhibition by DIDS (9, 26). The renal PAH/dicarboxylate exchanger is sensitive to inhibition by probenecid (18).
Fig 5.
INDY-mediated succinate transport is disulfonic stilbene-sensitive. Transport was almost completely inhibited by 0.1 mM 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), was modestly reduced by 1 mM phloretin or furosemide, and was unaffected by 1 mM probenecid.
INDY also Transports Citrate.
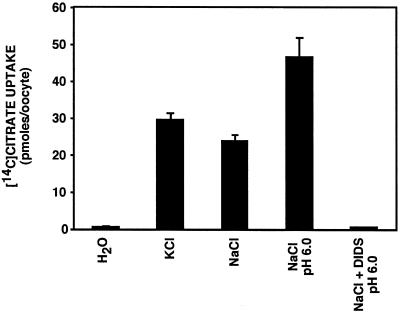
The inhibition of [14C]succinate uptake by citrate shown in Fig. 2 suggested that INDY can mediate the transport not only of dicarboxylates but also of the tricarboxylate citrate. To evaluate this possibility directly, we measured the uptake of [14C]citrate. As shown in Fig. 6, uptake of [14C]citrate was increased more than 50-fold in oocytes injected with Indy cRNA, as compared with water-injected controls. As in the case of succinate, uptake of [14C]citrate was sodium-independent. But, whereas transport of succinate was pH-independent (Fig. 4), uptake of [14C]citrate was markedly stimulated at pH 6.0 compared with that at pH 7.5 (Fig. 6). This result indicates either that citrate is preferentially transported in the dicarboxylate form or that the tricarboxylate form is cotransported with protons. As observed for succinate transport (Fig. 5), [14C]citrate uptake was sensitive to inhibition by the disulfonic stilbene DIDS (Fig. 6). It is interesting to note that although INDY has a Km for succinate in the range of high-affinity mammalian sodium-dicarboxylate cotransporters, pH-dependent citrate transport and pH-independent succinate transport have been generally observed as properties of low-affinity, rather than high-affinity, mammalian sodium-dicarboxylate cotransporters (17). Thus, these findings provide further evidence that INDY's combination of transport properties is unique.
Fig 6.
INDY mediates citrate transport. Compared with water-injected control, uptake of [14C]citrate was stimulated >50-fold into oocytes injected with Indy cRNA. Citrate uptake was not reduced by replacement of NaCl by KCl but was stimulated by imposition of an outside-acid pH gradient (pH 6.0). Citrate uptake was virtually abolished by 0.1 mM DIDS.
INDY Is Localized to the Plasma Membrane and Decreased in Indy Mutants.
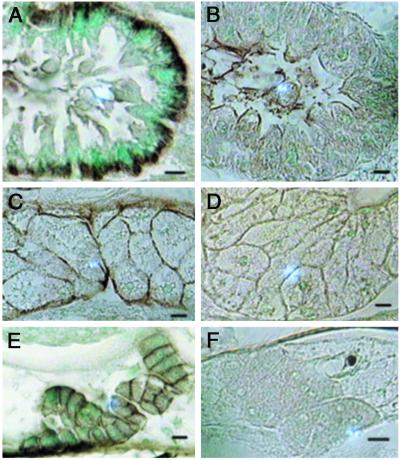
To gain additional insight into the physiological function of INDY, we identified its membrane sites of expression by immunocytochemical analysis using specific anti-peptide antibodies. INDY immunostaining was most prominently seen on the basolateral membrane of cells in the midgut (Fig. 7A) and the plasma membrane of fat body and oenocytes (Figs. 7 C and E). Animals with Indy mutations show a greatly diminished expression of anti-INDY staining in all of these tissues, demonstrating that the life-extending mutations in Indy cause a decrease in INDY activity (Figs. 7 B, D, and F). These findings confirm that INDY is a plasma membrane transporter in native tissues. Moreover, the polarized expression of INDY in the gut suggests that it may have a role in vectorial transport of Krebs cycle intermediates across the gut epithelium.
Fig 7.
Immunolocalization of INDY in control and mutant adult flies. Immunostaining of 5 μm paraffin sections of gut (A and B), fat body (C and D), and oenocytes (E and F) of control (A, C, E) and Indy mutant adult flies (B, D, F) by using rabbit anti-INDY primary antibody (1:5,000) and goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:200) and developed as described in Vectastain Elite ABC kit. Independent antibodies were raised to two different peptides of INDY. Both anti-peptide antibodies showed the same specificity. The preimmune rabbit antibody from each showed no specific staining on control tissue sections at 1:500–1:5,000. (Bars = 10 μm.)
Discussion
The functional expression studies in Xenopus oocytes indicate that INDY is a metabolite transporter that mediates the relatively high-affinity flux of dicarboxylates and citrate across the plasma membrane by a disulfonic stilbene-sensitive mechanism that is not coupled to Na+, K+, or Cl−. It is perhaps not surprising that INDY has unique transport properties because it shares only 34% amino acid identity with its closest homologs, the mammalian sodium-dicarboxylate cotransporters.
One possibility is that INDY mediates anion uniport, in which case it would mediate electrogenic dicarboxylate flux across the plasma membrane. This possibility is very unlikely, because INDY-mediated succinate uptake was insensitive to replacement of extracellular Na+ by K+. Because the plasma membrane of Xenopus oocytes has an appreciable conductance for K+, variations in the transmembrane gradient for this ion cause marked shifts in membrane potential (27). Thus, the lack of effect of external K+ on succinate uptake suggests an electroneutral mechanism of uptake. Indeed, in preliminary electrophysiologic studies, we were unable to detect current associated with INDY-mediated succinate transport in Xenopus oocytes (unpublished data). Given the lack of coupling to inorganic ions, it is most likely that the electroneutral process mediated by INDY is the exchange of organic anions across the plasma membrane, and that measured uptake of [14C]succinate and [14C]citrate into Xenopus oocytes occurs in exchange for endogenous Krebs cycle intermediates. Organic anion exchange can result in the net transfer of caloric or reducing equivalents across the plasma membrane when it involves pairs of Krebs cycle intermediates of unequal chain lengths (e.g., α-ketoglutarate vs. succinate) or different redox states (e.g., succinate vs. fumarate).
Previous studies have shown that Indy transcriptional activity is most prominent in tissues associated with the uptake, utilization, or storage of nutrients in the adult fly. These tissues include fat body, oenocytes, and a subset of midgut cells. Immunocytochemistry with anti-INDY peptide antibodies confirmed the tissue localization of INDY protein to these sites and demonstrated its association with the plasma membrane. In the midgut, most of the INDY staining was found on the basolateral region, suggesting that INDY is likely involved in the transepithelial transport of Krebs cycle intermediates from the lumen of the gut into the hemolymph.
Immunocytochemistry studies also revealed that the level of expression of INDY in these metabolically important tissues is diminished in Indy mutant animals. Based upon the site of insertion of the P element in each of the five Indy alleles, it had been postulated that the P element insertions would result in a decrease in Indy transcription. The P elements are not inserted in coding regions, so they should not lead to the expression of an abnormal INDY protein. Instead, the sites of insertion of the P elements in each of the mutants, either immediately upstream of the transcriptional start site or in the first intron upstream of the translational start site, suggest an affect on transcriptional regulation (probably a decrease in transcription). Northern blot hybridization supported the hypothesis that Indy transcriptional activity is diminished by showing a decrease in transcript levels in all Indy mutants tested (Indy302 and Indy206, unpublished data). The demonstration of a decrease in INDY protein levels in situ in the fat body, oenocytes, and midgut cells of Indy mutant animals by immunocytochemistry confirms the hypothesis that a decrease in INDY activity is causally related to life-span extension.
INDY appears to function as a sodium-independent mechanism for transporting Krebs cycle intermediates through the epithelium of the gut and across the plasma membranes of organs involved in intermediary metabolism and storage. A reduction in INDY activity by genetic mutations dramatically extends life span in fruit flies without sacrificing fertility or physical activity. Knowledge of INDY's function as a previously uncharacterized plasma membrane transporter of Krebs cycle intermediates in tissues participating in uptake, utilization, and storage of nutrients suggests potential therapeutic interventions for mimicking the life-extending effects of Indy mutations. Given the universal role of energy balance in aging (e.g., worms, flies, and rodents; refs. 1 and 2), genetic or pharmacological manipulation of functionally analogous transporters may provide a rational approach for extending healthy life in a variety of species, including humans.
Acknowledgments
We thank Friedrich Luft for support of F.K., Yohannes Hagos for providing us with fNaDC-3 cDNA, and Tyson Bross for assistance with the immunocytochemistry. This work was supported by National Institutes of Health Grants DK-17433 and DK-33793 (to P.S.A.), AG-14532 (to S.L.H.), and ES-11463 and AG-20816 (to B.R.), a Donaghue Investigator Award from The Patrick and Catherine Weldon Donaghue Medical Foundation (to S.L.H.), and an Ellison Medical Foundation Senior Investigator Award (to S.L.H). S.L.H. is a member of the Scientific Advisory Board of Elixir Pharmaceuticals, Inc., Cambridge, MA, which is dedicated to developing interventions to ameliorate the process of aging.
Abbreviations
Indy, I'm not dead yet
DIDS (4,4′-diisothiocyanostilbene-2,2′-disulfonic acid), PAH, p-aminohippurate
References
- 1.Masoro E. J. (2000) Exp. Gerontol. 35, 299-305. [DOI] [PubMed] [Google Scholar]
- 2.Finch C. E. & Ruvkun, G. (2001) Annu. Rev. Genomics Hum. Genet. 2, 435-462. [DOI] [PubMed] [Google Scholar]
- 3.Rogina B., Reenan, R. A., Nilsen, S. P. & Helfand, S. L. (2000) Science 290, 2137-2140. [DOI] [PubMed] [Google Scholar]
- 4.Kippen I., Hirayama, B., Klinenberg, J. R. & Wright, E. M. (1979) Proc. Natl. Acad. Sci. USA 76, 3397-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolffram S., Badertscher, M. & Scharrer, E. (1994) Exp. Physiol. 79, 215-226. [DOI] [PubMed] [Google Scholar]
- 6.Wright S. H. & Wunz, T. M. (1987) Am. J. Physiol. 253, F432-F439. [DOI] [PubMed] [Google Scholar]
- 7.Burckhardt G. (1984) Pflügers Arch. 401, 254-261. [DOI] [PubMed] [Google Scholar]
- 8.Edwards R. M., Stack, E. & Trizna, W. (1997) J. Pharmacol. Exp. Ther. 281, 1059-1064. [PubMed] [Google Scholar]
- 9.Pajor A. M. (2000) J. Membr. Biol. 175, 1-8. [DOI] [PubMed] [Google Scholar]
- 10.Moseley R. H., Jarose, S. & Permoad, P. (1992) Am. J. Physiol. 263, G871-G879. [DOI] [PubMed] [Google Scholar]
- 11.Ganapathy V., Ganapathy, M. E., Tiruppathi, C., Miyamoto, Y., Mahesh, V. B. & Leibach, F. H. (1988) Biochem. J. 249, 179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shank R. P. & Campbell, G. L. (1981) Life Sci. 28, 843-850. [DOI] [PubMed] [Google Scholar]
- 13.Trudeau M. C., Warmke, J. W., Ganetzky, B. & Robertson, G. A. (1995) Science 269, 92-95. [DOI] [PubMed] [Google Scholar]
- 14.Knauf F., Yang, C. L., Thomson, R. B., Mentone, S. A., Giebisch, G. & Aronson, P. S. (2001) Proc. Natl. Acad. Sci. USA 98, 9425-9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steffgen J., Burckhardt, B. C., Langenberg, C., Kuhne, L., Muller, G. A., Burckhardt, G. & Wolff, N. A. (1999) J. Biol. Chem. 274, 20191-20196. [DOI] [PubMed] [Google Scholar]
- 16.Tam J. P. (1988) Proc. Natl. Acad. Sci. USA 85, 5409-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajor A. M. (1999) Annu. Rev. Physiol. 61, 663-682. [DOI] [PubMed] [Google Scholar]
- 18.Van Aubel R. A., Masereeuw, R. & Russel, F. G. (2000) Am. J. Physiol. 279, F216-F232. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard J. B. & Miller, D. S. (1993) Physiol. Rev. 73, 765-796. [DOI] [PubMed] [Google Scholar]
- 20.Sekine T., Watanabe, N., Hosoyamada, M., Kanai, Y. & Endou, H. (1997) J. Biol. Chem. 272, 18526-18529. [DOI] [PubMed] [Google Scholar]
- 21.Reid G., Wolff, N. A., Dautzenberg, F. M. & Burckhardt, G. (1998) Kidney Blood Press. Res. 21, 233-237. [DOI] [PubMed] [Google Scholar]
- 22.Sweet D. H., Wolff, N. A. & Pritchard, J. B. (1997) J. Biol. Chem. 272, 30088-30095. [DOI] [PubMed] [Google Scholar]
- 23.Palmieri F., Prezioso, G., Quagliariello, E. & Klingenberg, M. (1971) Eur. J. Biochem. 22, 66-74. [DOI] [PubMed] [Google Scholar]
- 24.Crompton M., Palmieri, F., Capano, M. & Quagliariello, E. (1974) FEBS Lett. 46, 247-250. [DOI] [PubMed] [Google Scholar]
- 25.Fiermonte G., Palmieri, L., Dolce, V., Lasorsa, F. M., Palmieri, F., Runswick, M. J. & Walker, J. E. (1998) J. Biol. Chem. 273, 24754-24759. [DOI] [PubMed] [Google Scholar]
- 26.Chen X. Z., Shayakul, C., Berger, U. V., Tian, W. & Hediger, M. A. (1998) J. Biol. Chem. 273, 20972-20981. [DOI] [PubMed] [Google Scholar]
- 27.Dascal N. (1987) CRC Crit. Rev. Biochem. 22, 317-386. [DOI] [PubMed] [Google Scholar]