Abstract
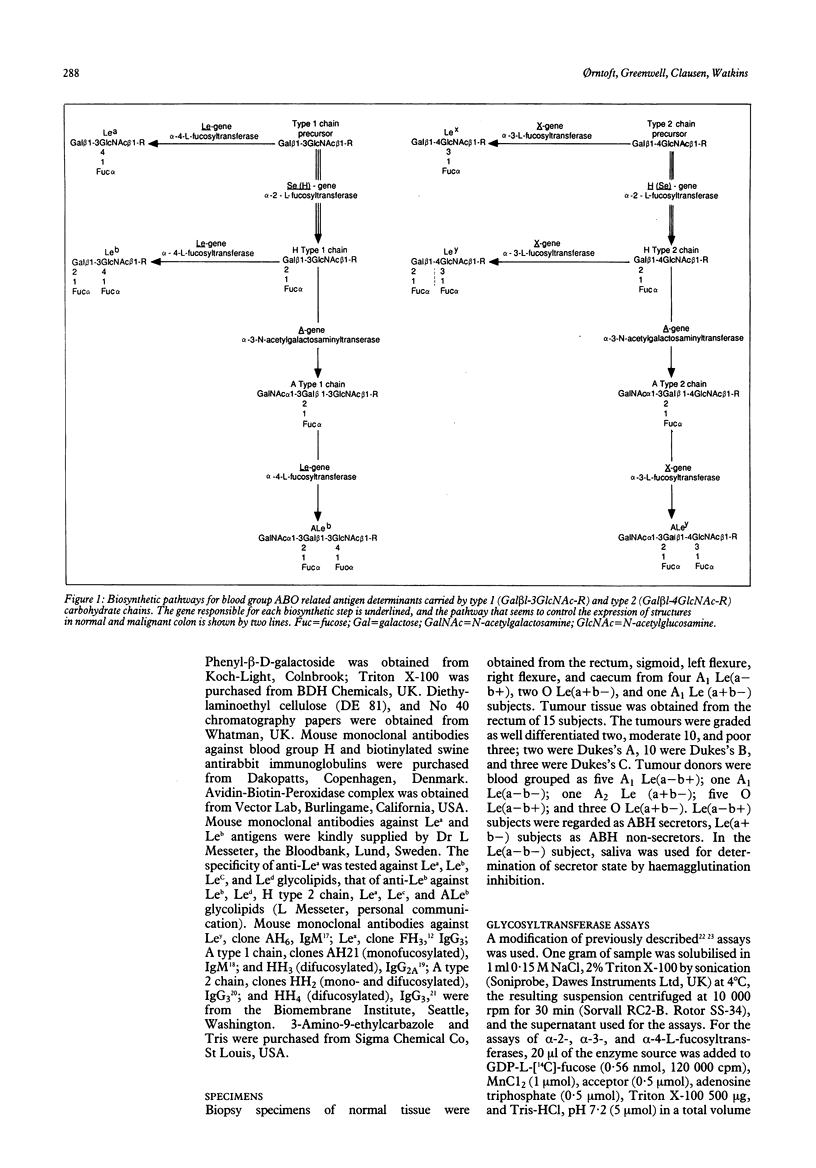
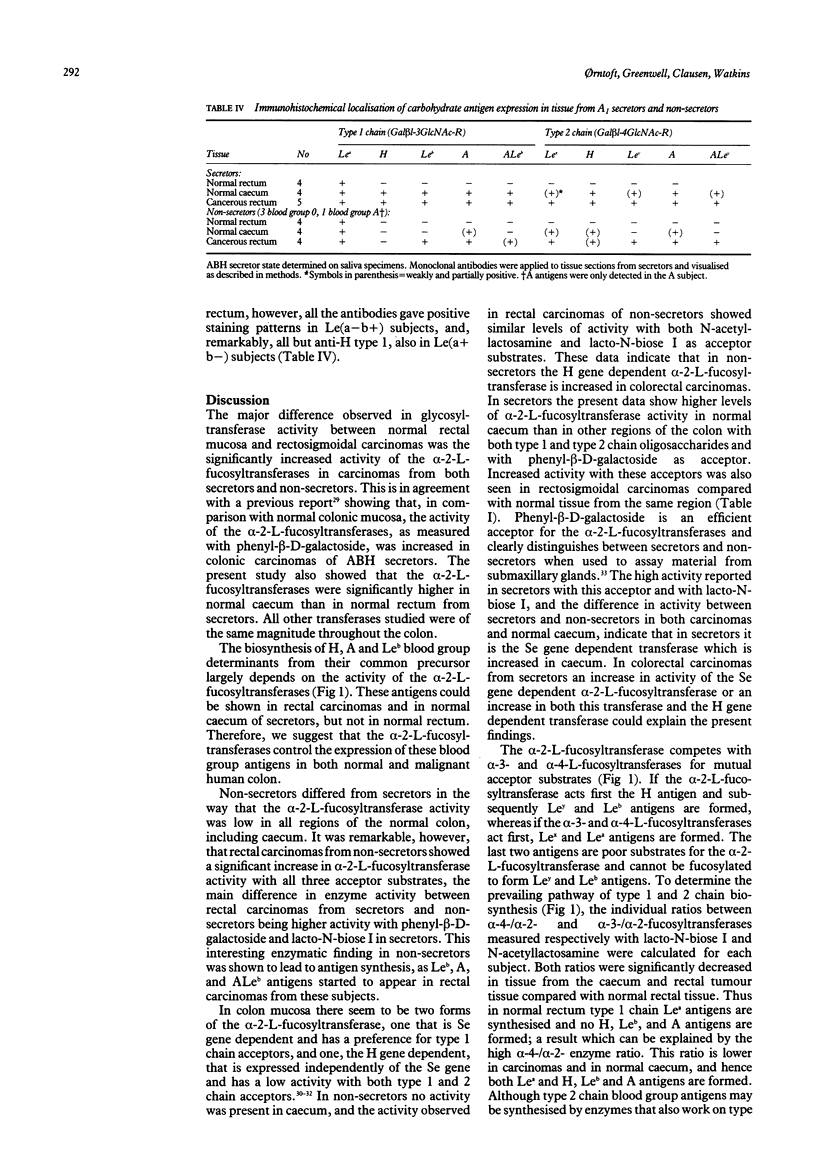
Blood group antigen expression in the distal human colon is related to the development of the organ and is modified by malignant transformation. To elucidate the biochemical basis for these changes, we have (a) analysed the activity of glycosyltransferases coded for by the H, Se, Le, X, and A genes, in tissue biopsy specimens from normal and malignant proximal and distal human colon; (b) characterised the glycosphingolipids expressed in the various regions of normal and malignant colon by immunostaining of high performance thin layer chromatography plates; and (c) located the antigens on tissue sections from the same subjects by immunohistochemistry. In both secretors and non-secretors we found a significantly higher activity of alpha-2-L-fucosyltransferases in carcinomatous rectal tissue than in tissue from normal subjects, whereas the other transferase activities studied showed no significant differences. The acceptor substrate specificity suggested that both the Se and the H gene dependent alpha-2-L-fucosyltransferases are increased in carcinomas. In non-malignant tissue the only enzyme which showed appreciably higher activity in caecum than in rectum was alpha-2-L-fucosyltransferase. Immunochemistry and immunohistochemistry showed alpha-2-L-fucosylated structures in normal caecum from secretors and in tumour tissue from both secretors and non-secretors. We conclude that the alpha-2-L-fucosyltransferases control the expression of ABH, and Lewis(b) structures in normal and malignant colon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Levery S. B., Hakomori S. The antibody specific to type 1 chain blood group A determinant. J Immunol. 1984 Apr;132(4):1951–1954. [PubMed] [Google Scholar]
- Abe K., McKibbin J. M., Hakomori S. The monoclonal antibody directed to difucosylated type 2 chain (Fuc alpha 1 leads to 2Gal beta 1 leads to 4[Fuc alpha 1 leads to 3]GlcNAc; Y Determinant). J Biol Chem. 1983 Oct 10;258(19):11793–11797. [PubMed] [Google Scholar]
- Clausen H., Levery S. B., McKibbin J. M., Hakomori S. Blood group A determinants with mono- and difucosyl type 1 chain in human erythrocyte membranes. Biochemistry. 1985 Jul 2;24(14):3578–3586. doi: 10.1021/bi00335a028. [DOI] [PubMed] [Google Scholar]
- Clausen H., Levery S. B., Nudelman E., Baldwin M., Hakomori S. Further characterization of type 2 and type 3 chain blood group A glycosphingolipids from human erythrocyte membranes. Biochemistry. 1986 Nov 4;25(22):7075–7085. doi: 10.1021/bi00370a048. [DOI] [PubMed] [Google Scholar]
- Clausen H., McKibbin J. M., Hakomori S. Monoclonal antibodies defining blood group A variants with difucosyl type 1 chain (ALeb) and difucosyl type 2 chain (ALey). Biochemistry. 1985 Oct 22;24(22):6190–6194. doi: 10.1021/bi00343a024. [DOI] [PubMed] [Google Scholar]
- Dabelsteen E., Buschard K., Hakomori S., Young W. W. Pattern of distribution of blood group antigens on human epidermal cells during maturation. J Invest Dermatol. 1984 Jan;82(1):13–17. doi: 10.1111/1523-1747.ep12258874. [DOI] [PubMed] [Google Scholar]
- Dabelsteen E., Vedtofte P., Hakomori S. I., Young W. W. Carbohydrate chains specific for blood group antigens in differentiation of human oral epithelium. J Invest Dermatol. 1982 Jul;79(1):3–7. doi: 10.1111/1523-1747.ep12510411. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Fukushi Y., Hakomori S., Nudelman E., Cochran N. Novel fucolipids accumulating in human adenocarcinoma. II. Selective isolation of hybridoma antibodies that differentially recognize mono-, di-, and trifucosylated type 2 chain. J Biol Chem. 1984 Apr 10;259(7):4681–4685. [PubMed] [Google Scholar]
- Fukushima K., Hirota M., Terasaki P. I., Wakisaka A., Togashi H., Chia D., Suyama N., Fukushi Y., Nudelman E., Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984 Nov;44(11):5279–5285. [PubMed] [Google Scholar]
- Holmes E. H., Ostrander G. K., Clausen H., Graem N. Oncofetal expression of Lex carbohydrate antigens in human colonic adenocarcinomas. Regulation through type 2 core chain synthesis rather than fucosylation. J Biol Chem. 1987 Aug 15;262(23):11331–11338. [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Fukushi Y., Palekar A., Phelps P. C., Shamsuddin A. M., Trump B. F., Hakomori S., Kim Y. S. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 1986 May;46(5):2627–2632. [PubMed] [Google Scholar]
- Kaizu T., Levery S. B., Nudelman E., Stenkamp R. E., Hakomori S. Novel fucolipids of human adenocarcinoma: monoclonal antibody specific for trifucosyl Ley (III3FucV3FucVI2FucnLc6) and a possible three-dimensional epitope structure. J Biol Chem. 1986 Aug 25;261(24):11254–11258. [PubMed] [Google Scholar]
- Kannagi R., Fukushi Y., Tachikawa T., Noda A., Shin S., Shigeta K., Hiraiwa N., Fukuda Y., Inamoto T., Hakomori S. Quantitative and qualitative characterization of human cancer-associated serum glycoprotein antigens expressing fucosyl or sialyl-fucosyl type 2 chain polylactosamine. Cancer Res. 1986 May;46(5):2619–2626. [PubMed] [Google Scholar]
- Kim Y. S., Yuan M., Itzkowitz S. H., Sun Q. B., Kaizu T., Palekar A., Trump B. F., Hakomori S. Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res. 1986 Nov;46(11):5985–5992. [PubMed] [Google Scholar]
- Kumazaki T., Yoshida A. Biochemical evidence that secretor gene, Se, is a structural gene encoding a specific fucosyltransferase. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4193–4197. doi: 10.1073/pnas.81.13.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pendu J., Oriol R., Juszczak G., Liberge G., Rouger P., Salmon C., Cartron J. P. alpha-2-L-fucosyltransferase activity in sera of individuals with H-deficient red cells and normal H antigen in secretions. Vox Sang. 1983;44(6):360–365. doi: 10.1111/j.1423-0410.1983.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Oriol R., Le Pendu J., Mollicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51(3):161–171. doi: 10.1111/j.1423-0410.1986.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Orntoft T. F., Wolf H., Watkins W. M. Activity of the human blood group ABO, Se, H, Le, and X gene-encoded glycosyltransferases in normal and malignant bladder urothelium. Cancer Res. 1988 Aug 1;48(15):4427–4433. [PubMed] [Google Scholar]
- SZULMAN A. E. THE HISTOLOGICAL DISTRIBUTION OF THE BLOOD GROUP SUBSTANCES IN MAN AS DISCLOSED BY IMMUNOFLUORESCENCE. III. THE A, B, AND H ANTIGENS IN EMBRYOS AND FETUSES FROM 18 MM IN LENGTH. J Exp Med. 1964 Apr 1;119:503–516. doi: 10.1084/jem.119.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Schachter H., Michaels M. A., Tilley C. A., Crookston M. C., Crookston J. H. Qualitative differences in the N-acetyl-D-galactosaminyltransferases produced by human A1 and A2 genes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):220–224. doi: 10.1073/pnas.70.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears H. F., Herlyn M., Del Villano B., Steplewski Z., Koprowski H. Monoclonal antibody detection of a circulating tumor-associated antigen. II. A longitudinal evaluation of patients with colorectal cancer. J Clin Immunol. 1982 Apr;2(2):141–149. doi: 10.1007/BF00916898. [DOI] [PubMed] [Google Scholar]
- Szulman A. E., Marcus D. M. The histologic distribution of the blood group substances in man as disclosed by immunofluorescence. VI. The Le and Le antigens during fetal development. Lab Invest. 1973 May;28(5):565–574. [PubMed] [Google Scholar]
- Watkins W. M., Greenwell P. Biosynthesis of blood group ABH antigens: genetic regulation and tissue distribution. Transplant Proc. 1987 Dec;19(6):4413–4415. [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]
- Yuan M., Itzkowitz S. H., Palekar A., Shamsuddin A. M., Phelps P. C., Trump B. F., Kim Y. S. Distribution of blood group antigens A, B, H, Lewisa, and Lewisb in human normal, fetal, and malignant colonic tissue. Cancer Res. 1985 Sep;45(9):4499–4511. [PubMed] [Google Scholar]



