Abstract
Rheumatoid arthritis (RA) is a chronic, systemic, and inflammatory disease of connective tissue with unknown etiology. We investigated whether aberrant immune responses to glycosaminoglycans (GAGs), a major component of joint cartilage, joint fluid, and other soft connective tissue, causes this disease. Here we show that injection of GAGs such as hyaluronic acid, heparin, and chondroitin sulfates A, B, and C induce arthritis, tendosynovitis, dermatitis, and other pathological conditions in mice. We developed a technique by staining tissue specimens with fluorochrome- or biotin-labeled GAGs to visualize the direct binding between cells and GAGs. We discovered that inflammatory infiltrates from the affected tissue are dominated by a distinct phenotype of GAG-binding cells, a significant portion of which are CD4+ T cells. GAG-binding cells seem to be expanded in bone marrow of GAG-immunized mice. Furthermore, we identified GAG-binding cells in inflamed synovial tissue of human patients with RA. Our findings suggest that carbohydrate self-antigenic GAGs provoke autoimmune dysfunctions that involve the expansion of GAG-binding cells which migrate to anatomical sites rich in GAGs. These GAG-binding cells might, in turn, promote the inflammation and pathology seen both in our murine model and in human RA.
Autoimmune diseases of connective tissue, a group of diverse diseases of unknown etiology, include rheumatoid arthritis (RA), systemic lupus erythematosus, progressive systemic sclerosis or systemic scleroderma, polymyositis, dermatomyositis, and Sjögren syndrome (1–3). They share extensive, overlapping clinical, laboratory, and pathological features, especially during the early stages, often making classification and diagnosis difficult (1–3). The most common disease of this group is RA, a chronic inflammatory disease that attacks primarily the joints but may extend to connective tissue throughout the body (1–3). These conditions affect people of all ages and frequently cause disability and chronic impairments (2). Despite important advances in understanding many pathogenetic aspects, the etiologies of autoimmune connective tissue diseases remain a longstanding medical mystery.
Connective tissue comprises thin layers of cells separated by extracellular matrices, which contain primarily proteoglycans consisting of glycosaminoglycans (GAGs) covalently linked to tissue-specific core proteins (4, 5). GAGs include hyaluronic acid (HA), chondroitin sulfate A (CSA), B (CSB), and C (CSC), heparin (HP), heparan sulfate, and keratan sulfate (4). They are a family of highly anionic polysaccharides with similar disaccharide repeating units of uronic acid and hexosamine (4). Changes in the levels or molecular nature of GAGs have been previously associated with some connective tissue diseases. For example, patients with RA and scleroderma have elevated concentrations of GAGs in blood and synovial fluid, and destruction of involved joints in RA patients correlates positively with high GAG levels in synovial fluid (5–7). Despite these findings, aberrant immune responses to GAGs have not been examined as a possible cause of RA or other related diseases.
Carbohydrates are generally considered inert or poor immunogens that do not elicit cellular and mature humoral responses. This perception may have precluded the investigation of GAGs as possible antigens associated with autoimmune diseases. However, it is well known that GAG-rich extracellular matrices are reservoirs for growth factors and other agents that control cell behavior and that GAGs interact with various proteins and regulate cell development, adhesion, differentiation, and proliferation (8–12). Given the diverse biological activities of GAGs, their close association with RA and related diseases, and the abundance of GAGs in connective tissue, we hypothesized that an aberrant immune response to GAGs might play a role in connective tissue diseases. Here we show that administration of GAGs causes an autoimmune connective tissue disease in mice and investigate its significance for human RA.
Materials and Methods
Materials.
HA, HP, CSA, CSB, and CSC were purchased from Sigma-Aldrich and purified by digestion with DNase I, RNase A, and proteinase K (Worthington) and fractionation on a Superdex 200 column (Amersham Pharmacia). The average molecular masses of HA, HP, CSA, CSB, and CSC were 1,100, 59, 114, 100, and 970 kDa, respectively. GAGs were free of protein and nucleic acids as verified by 1H NMR spectroscopy at 500 MHz, UV-visible scanning from 190 to 300 nm, and Bradford protein assay (13). Fluorescein-labeled GAGs were prepared as described (14). To prepare biotin-labeled GAGs, 10 mg of GAG dissolved in 0.2 ml of 0.1 M Mes buffer (pH 5) were mixed with 0.3 ml of 50 mM biotin hydrazide and 10 mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma-Aldrich). The mixture was stirred at room temperature for 16 h and then desalted on a PD-10 column (Amersham Pharmacia). The resultant GAG-biotin products were structurally confirmed by 1H NMR spectroscopy.
Mouse Model.
Groups of 8–12 female BALB/c mice (The Jackson Laboratory), 6–8 weeks old, were injected intradermally at the base of the tail with 100 μg of GAGs dissolved in 25 μl of PBS (50 mM phosphate/0.15 M NaCl, pH 7.2) and mixed with an equal volume of 5% Al(OH)3 adjuvant (Superfos Biosector, Frederikssund, Denmark). Control mice received PBS and Al(OH)3 only. Injections were given on days 1, 16, 43, 80, and 100, respectively. Mouse sera were obtained on days −1, 8, 15, 37, 75, and 118. Mice were examined every other day for erythema and paw swelling. The symptoms were scored from 0 to 3 for all four paws according to the severity of erythema and swelling. A score of 0 indicated no evidence of erythema and paw swelling, 1 indicated erythema and subtle swelling, 2 indicated erythema and obvious swelling, and 3 indicated erythema and severe paw swelling. To avoid ambiguity, we considered a mouse sick if it had at least one paw scored at 2 or higher. At various time points, mice from every group were euthanized to permit histological analysis. Mice were fixed in Bouins' fixative, their bones were decalcified, specimens were embedded in paraffin, and thin sections were stained with hematoxylin and eosin.
GAG-Fluorescence Staining.
Paraffin-embedded tissue sections were immersed twice for 10 min in xylene, twice for 3 min in 100% ethanol, and three times for 5 min in PBS. The sections were blocked with PBS containing 1% BSA and 5% FCS (Life Technologies, Grand Island, NY) for 1 h at 25°C. Each slide was incubated with 100 μl of 0.5 mg⋅ml−1 fluorescein-labeled HA (5 mol % of fluorescein) or biotin-labeled HP, CSA, CSB, or CSC (5–10 mol % of biotin) at 25°C for 2 h. Slides incubated with biotin-GAGs were treated with 100 μl of 0.1 mg⋅ml−1 Alexa Fluor 568-labeled streptavidin (Molecular Probes) at 25°C for 3 h. For costaining experiments, tissue sections were incubated first with 100 μl of 0.1 mg⋅ml−1 biotin-labeled rat anti-mouse CD4 or CD44 antibodies (Southern Biotechnology Associates) at 4°C overnight and then with 100 μl of 0.1 mg⋅ml−1 Alexa Fluor 568-labeled streptavidin mixed with 0.5 mg⋅ml−1 HA-fluorescein at 25°C for 3 h.
Quantitation of Antibodies.
The concentrations of serum antibodies against GAGs in immunized mice were determined by quantitative ELISAs (15). EIA/RIA 96-well plates (Corning) were coated with 100 μl of 50 μg⋅ml−1 GAG in 0.1 M NaHCO3 buffer (pH 8.6) at 4°C for 16 h. The plates were washed with PBS+ (50 mM phosphate/0.15 M NaCl/1 mM CaCl2/1 mM MgCl2/0.05% Brij 35, pH 7.4) three times and then blocked with 5% FCS in PBS+ at 25°C for 2 h. Mouse sera diluted 1:50 in incubation buffer (PBS+, 1% BSA, pH 7.4) were added to wells in duplicates and incubated at 25°C for 2 h. The plates were then washed and incubated with 0.5 μg⋅ml−1 of alkaline phosphatase-labeled goat anti-mouse IgM or IgG (Southern Biotechnology Associates) in incubation buffer at 25°C for 2 h. The plates were developed with 1 mg⋅ml−1 p-nitrophenylphosphate in 1 M Tris with 0.3 mM MgCl2 (pH 9.8) at 25°C. The concentrations of antibodies against GAGs were determined by comparison of the optical densities of test sera at 405 nm with those of standards developed on the same plate by using known concentrations of purified mouse IgM or IgG.
Cell Proliferation Assays.
Cell assays were performed in 96-well cell culture plates (Corning). Suspensions of mouse splenocytes were prepared from freshly removed mouse spleens and erythrocytes were depleted by ACK lysing buffer (0.15 M NH4Cl/10 mM KHCO3/0.1 mM Na2EDTA, pH 7.2). A portion of splenocytes was fractionated on a nylon wool column to obtain B and T cell-enriched subpopulations (16, 17). Splenocytes (2.6 × 106 ml−1) and B cell-enriched fractions (1.8 × 106 ml−1) were cultured with 20 μg⋅ml−1 of CSA, CSB, CSC, HP, or HA in RPMI medium 1640 (Invitrogen) supplemented with 10% FCS (RPMI 1640–10). T cell-enriched fractions (2.2 × 106 ml−1) were cultured with irradiated splenocytes (1.1 × 106 ml−1) as antigen-presenting cells and 20 μg⋅ml−1 GAG in RPMI 1640–10 medium. To obtain more specific cell types, splenocytes were separated by BD Imag anti-mouse CD4 or CD45R/B220 particles (BD Biosciences, San Diego) with a magnet into four fractions: CD4+, CD4−, B220+, and B220−. Each fraction (2–3 × 106 ml−1) was cultured with individual GAGs in RPMI 1640–10 medium. Con A and lipopolysaccharide from Escherichia coli (Sigma-Aldrich) were used as positive controls and RPMI 1640–10 medium alone served as a negative control. All cells were cultured for 4–9 days. Cell proliferation was measured as incorporation of 5-bromo-2′-deoxyuridine (BrdUrd) into DNA during the final 16 h of culture and detected with alkaline phosphatase-labeled anti-BrdUrd by ELISA. Cells were monitored for expression of CD4 and CD44 by fluorescence-activated cell sorter analyses.
Results
Animal Model.
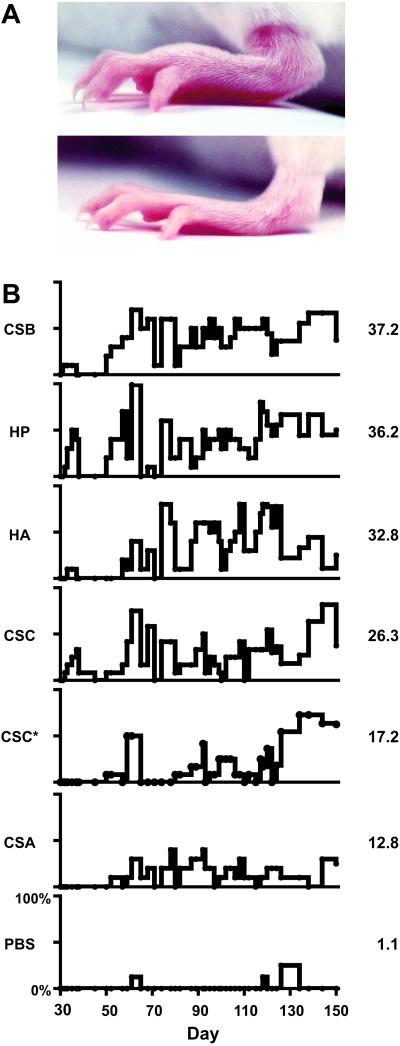
To study the consequences of immunization with GAGs, we developed a mouse model that involved injecting GAGs into BALB/c mice with or without adjuvant Al(OH)3. We tested CSA, CSB, CSC, HP, and HA mixed with Al(OH)3 and also CSC alone without adjuvant. Each mouse received five injections. After the second injection, mice started to show symptoms in their paws on day 31. All GAGs induced symptoms of swollen paws, edema, and erythema of paws and ears (Fig. 1). Both front and rear paws were affected and loss of hair from paws was obvious in some sick mice. The symptoms were fluctuating. The mice showed symptoms for a few days, recovered for a few days, and then became sick again. Overall, mice treated with GAGs became chronically sick, showing on-and-off symptoms for months (Fig. 1). Disease frequency and severity increased over time. Exacerbation and remission of the symptoms resemble the course of human RA (1–3). The average percentage of mice that were sick per day (prevalence) was greatest with CSB (37.2%), followed by HP (36.2%), HA (32.8%), CSC (26.3%), CSC* (17.2%, given without adjuvant), CSA (12.8%), and control PBS/adjuvant (1.1%).
Fig 1.
(A) Examples of a swollen, erythematous rear paw from a mouse treated with CSC (Top) and a normal rear paw from a PBS control mouse (Bottom), both at day 60 of the experiment. Note the involvement of tarsal, metatarsal, and phalangeal joints. (B) Time courses of disease prevalence for mice treated with GAGs or PBS as control. CSC* denotes CSC treatment without Al(OH)3 adjuvant. Ordinates range from 0 to 100% for each graph. Numbers to the right of each graph represent average percentages of diseased mice per day. Note the fluctuating nature of disease progression.
Histopathology.
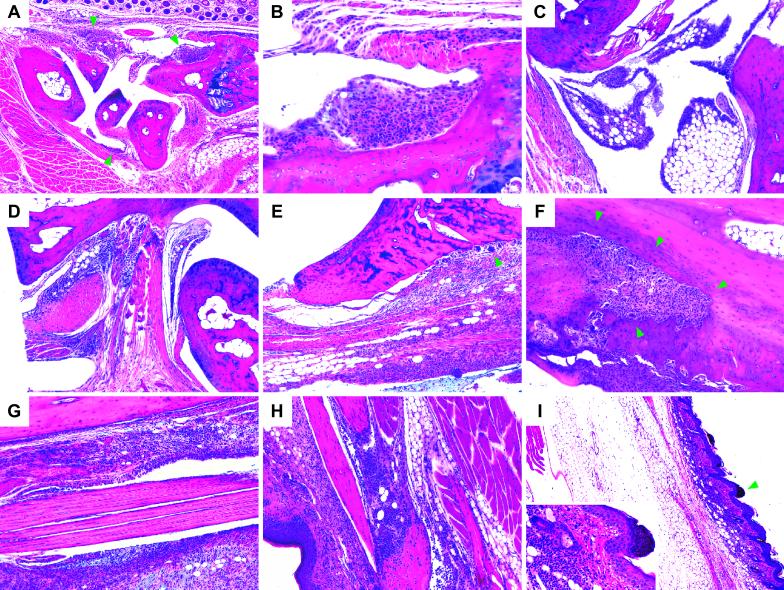
Sick mice treated with different GAGs showed very similar pathological changes, including synovial and s.c. edema, hyperplasia and hypertrophy of synovial lining cells, vascular congestion and dilation, and cellular infiltrates in various connective tissues (Fig. 2). Normal synovium or tendon sheaths consist of a thin layer of synoviocytes that line the joint cavity and rest on connective tissue and fat (3). The synovium of sick mice is hyperplastic and thickened by infiltrating lymphocytes and macrophages (Fig. 2). Abnormally large numbers of cells infiltrated synovial membranes and tendon sheaths of various distal joints in the paws, such as carpal/tarsal joints and metacarpal/metatarsal/phalangeal joints. After 4 months, epiphysial bone erosion was obvious in several sick mice (Fig. 2).
Fig 2.
(A) Sagittal section through a metacarpus demonstrating global synovial hyperplasia and hypertrophy (focal examples indicated by arrowheads), marked periarthritis, and tendosynovitis. Distal radius to the right; dorsal hair follicles along upper edge. (B) Hyperplastic and hypertrophic (eosinophilic) synovium with lymphoplasmocytic cell infiltration (magnification from A near upper right arrowhead). (C) Hyperplastic and hypertrophic synovia on the dorsal side of an ankle joint. Tibia and calcaneus near upper and right edges, respectively. (D) Pronounced dorsal periarthritis near talocalcaneal and transverse tarsal joints. Talus and calcaneus along lower right and upper edges, respectively. (E) Cell infiltration near distal epiphysis of the tibia with involvement of the extensor tendon sheath. Beginning, pannus-like epiphysial bone erosion involving multinucleate giant cells (arrowhead). (F) Advanced, pannus-like osteo- and chondrolytic lesion in the distal tibia (arrowheads) involving numerous multinucleate giant cells. Marrow cavity in upper right corner. (G) Severe peritendinitis in the tibial extensor compartment. (H) Peritendinitis and dermal cell infiltration near a distal interphalangeal joint. Palmar epidermis to the left. (I) Marked s.c. edema and dermatitis distally in a rear paw. (Inset) Magnification of an area (arrow) with dermal lymphoplasmocytic cell infiltration and parakeratosis. A and B, C and I, and D–H are from groups CSC*, HA, and CSC, respectively. Mice from other GAG groups exhibit similar histopathology.
In addition to synovitis and tendosynovitis, GAGs also caused dermatitis. The dermis in the distal extremities was infiltrated by significant numbers of inflammatory cells, predominantly lymphocytes (Fig. 2). Epidermal thickening and parakeratosis were commonly visible. However, the skin over the injection site in the tails appeared normal. Macro- and histopathologic examination of large internal organs such as the lungs, liver, heart, kidneys, and brain showed no abnormalities. Enlarged, hyperplastic popliteal lymph nodes were found in several sick mice (Fig. 3). The general scarcity of neutrophils suggests that the inflammatory response was not due to acute infectious processes. Overall, the pathological changes we observed in GAG-immunized sick mice (e.g., synovitis, tendosynovitis, and dermatitis) are frequently observed in human patients with RA and several other connective tissue diseases (1, 3).
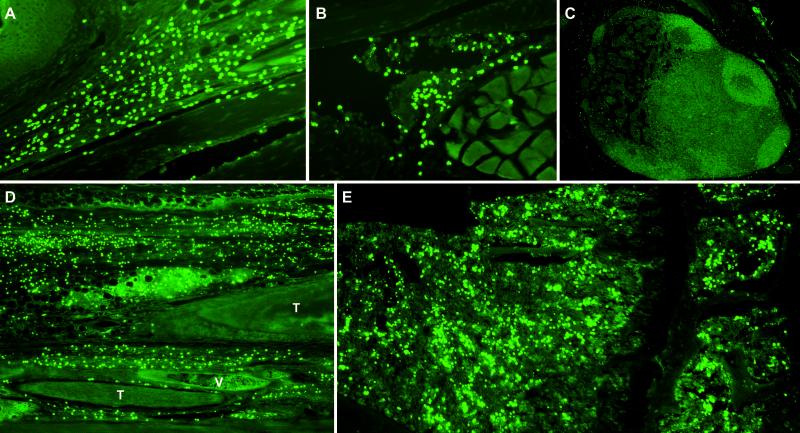
Fig 3.
GAG staining of tissues from GAG-treated mice demonstrating HA-binding cells. (A) Dermal GAG-binding cell infiltration near a distal interphalangeal joint. Note the epidermis (upper left) and a flexor tendon (bottom). (B) GAG-binding cells in hyperplastic synovium. (C) Scarcity of GAG-binding cells in an activated popliteal lymph node. (D) Connective tissue and tendon sheath infiltration in the extensor compartment near a talocalcaneal joint. T, tendon; V, vein. (E) GAG-binding cells in proximal epiphysial bone marrow of the tibia.
Autoantibodies.
To test whether these mice developed autoantibodies against GAGs, we measured serum levels of GAG-specific IgM and IgG by ELISA. Mice treated with GAGs developed an average IgM concentration of 0.19 μg·ml−1 but much lower levels of IgG (<1 ng·ml−1). However, only slightly lower amounts of GAG-specific IgM antibodies were also detected in control mice. In addition, sera from different groups cross-reacted with other GAGs. Overall, GAGs induced an IgM-dominated antibody response and did not induce an antibody isotype switch from IgM to IgG even after five doses of GAG immunization. These results indicate that the humoral response to GAGs is typical of polysaccharide antigens that induce antibodies by means of a T cell-independent mechanism (18). However, we did not observe a consistent GAG-specific antibody response that correlated with the most potent disease-inducing GAG antigens in our mouse model. Furthermore, although disease severity increased over time, GAG-specific antibody levels did not change significantly.
We also examined rheumatoid factor, that is, autoantibodies that recognize the Fc portion of IgG (19). Although its role in the pathogenesis of RA is not fully understood and many healthy people also express rheumatoid factor, most patients with RA have elevated levels of rheumatoid factor (19). We tested sera from all groups of mice by ELISA by using plates coated with mouse IgG. We detected only very small amounts of IgG-binding IgM (<6 ng·ml−1), and the amount of rheumatoid factor in sera from GAG-treated mice was not significantly different from that in control mice.
Cellular Effects.
GAGs display complex biological activities toward various cells, including lymphocytes, monocytes, dendritic, and stromal cells (10, 17, 20–23). We examined cellular responses to GAGs to identify a correlation with disease development in mice. We cultured unfractionated and fractionated mouse splenocytes ex vivo with pure CSA, CSB, CSC, HP, and HA. At 20 μg·ml−1, CSB increased splenocyte proliferation 3.17-fold over control after 6 days of culture. HP, CSA, HA, and CSC increased splenocyte proliferation 1.60-, 1.46-, 1.14-, and 1.12-fold, respectively. With B cell-enriched splenocytes, the proliferative activity followed the order of CSB, HP, CSA, CSC, and HA (from greatest to least). T cell-enriched splenocytes were cultured with both GAGs and irradiated splenocytes. Proliferative activity followed the order of CSB, HP, HA, CSC, and CSA (from greatest to least). Assays on isolated CD4+, CD4−, B220+, and B220− splenocytes revealed that GAGs stimulate B220-depleted splenocytes, which include T lymphocytes and monocytes, the most. These results and further fluorescence-activated cell sorter analyses (data not shown) indicate that GAGs differentially activate the replication of various cell types in a complex manner. CSB is the most active GAG in all cell proliferation assays and also in inducing disease in mice (Fig. 1). Moreover, the order of proliferative potency of GAGs on T cell-enriched splenocytes is positively correlated with disease prevalence (CSB > HP > HA > CSC > CSA; Fig. 1). These findings suggest that a T cell-mediated response may be involved in disease development in GAG-immunized mice.
GAG Binding as Distinct Phenotype of Infiltrating Cells.
Because inflammatory cells preferentially accumulated in connective tissue where GAGs are abundant, we hypothesized that these cells express either high-affinity and/or large amounts of GAG-binding receptors. To test this hypothesis, we developed a technique by staining thin sections of fixed mouse tissue with fluorescein- or biotin-labeled GAGs. Strikingly, the great majority of infiltrating cells in tendon sheaths, synovial membranes, and s.c. spaces bind HA (Fig. 3). Fluorescence staining with biotin-labeled HP or chondroitin sulfates and fluorochrome-labeled streptavidin confirmed that the infiltrating cells also bind these GAGs.
Because CD44 is a ubiquitous receptor for HA and other GAGs (24, 25), we tested whether CD44 is expressed on infiltrating cells by immunofluorescence costaining with anti-CD44 and fluorescein-labeled HA. A significant portion of cells observed were CD44+ and also bind HA (data not shown). Antibodies to CD44 did not reduce or block the binding of HA to the majority of the infiltrating cells, suggesting that the infiltrating cells might be quite heterogeneous or that these cells express multiple receptors for GAGs. Nonetheless, fluorescence-activated cell sorter analyses revealed that CSB-, HP-, and HA-cultured mouse splenocytes express more CD44 than cells cultured without GAGs (data not shown). These results indicate that GAGs can up-regulate CD44, which might, in turn, be involved in development of the GAG-induced disease observed in mice.
Infiltrating CD4+ T Cells Bind GAGs.
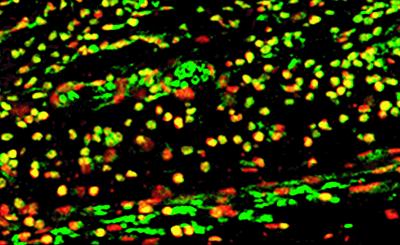
T cells are thought to play crucial roles in many autoimmune diseases. For example, CD4+ T cells are the largest subpopulation of mononuclear cells and contribute the majority of lymphocytes to synovial tissue in human RA and other autoimmune diseases (26–29). To identify CD4+ T cells in sick mice, we stained sections of their paws with biotin-labeled rat anti-mouse CD4 antibodies and fluorescein-labeled streptavidin. Indeed, large portions of the cell infiltrates in synovial membranes, tendon sheaths, and s.c. spaces were CD4+ T cells. We then investigated whether the infiltrating CD4+ T cells also bind GAGs. We costained the tissue sections with biotin-labeled CD4 antibodies plus Alexa Fluor 568-labeled streptavidin and HA-fluorescein. The costaining revealed that infiltrating CD4+ cells indeed bind HA (Fig. 4). To test whether HA binds CD4 directly, we blocked sections with monoclonal CD4 antibodies and then stained with HA-fluorescein. Antibodies to CD4 did not inhibit the binding of HA to CD4 cells. These findings indicate that HA may bind other receptors on the T cell surface or at least that the binding sites on CD4 are different.
Fig 4.
Immunostaining showing GAG-binding CD4+ T cells. CD4+ T cells are red, HA-binding cells are green, and costaining is yellow.
Expansion of GAG-Binding Cells in Bone Marrow.
Although much information has been gained on lymphocytes accumulated in joint tissue, virtually nothing is known about the events preceding their arrival from the bloodstream. Histologically, the bone marrow of several sick mice seemed hyperplastic. We examined bone marrow specimens by staining with HA-fluorescein. Surprisingly, a large number of GAG-binding cells were found in the bone marrow (Fig. 3). A portion of these GAG-binding marrow cells are CD4+ T cells (data not shown). We also examined the bone marrow of control mice but found only a very small number of HA-binding cells. Although several sick mice had grossly enlarged lymph nodes, very few GAG-binding cells were trafficking inside (Fig. 3). Hence, the expansion of autoreactive GAG-binding cells did not occur in the lymph nodes. Previous findings also indicate that infiltrating T cells are not expanded locally in the joints of arthritic patients (26, 27). T cell cytokines, especially Th2 cytokines, are almost completely absent in the human rheumatic joint and Th1 cytokines seem to be produced at only low levels compared with those in other diseases of chronic inflammation (27). Thus, our finding that GAG-binding cells are expanded in bone marrow may help clarify the paradox of the origination of T and other inflammatory cells that migrate to joints.
GAG-Binding Cells in Patients with RA.
To investigate the relevance of GAGs and GAG-binding cells in human patients with RA, we stained surgical tissue specimens from several patients with fluorescein- or biotin-labeled HA, HP, and CSB. We observed that a significant number of infiltrating cells bind GAGs in patients with RA (Fig. 5). Normal synovial or traumatic-arthrotic tissue did not show GAG binding (data not shown). Costaining for CD4 revealed that a significant portion but not all GAG-binding cells are CD4+ T cells (Fig. 5). These findings demonstrate that infiltrating cells in the specimens from human RA patients display GAG-binding properties very similar to diseased tissues from mice immunized with GAGs.
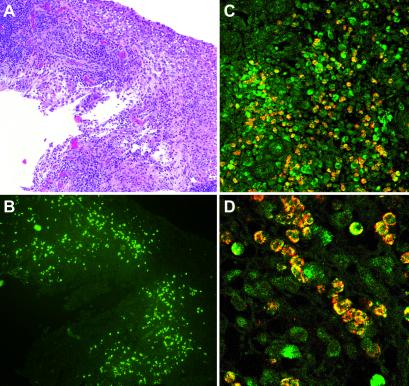
Fig 5.
Left knee synovial tissue specimen from a 33-year-old female patient with RA. (A) Inflamed and hyperplastic synovium with lymphoplasmocytic infiltration (hematoxylin and eosin staining). (B) GAG staining showing HA-binding cell infiltrates (neighboring section from A). (C and D) Costaining for HA-binding CD4+ T cells. CD4+ T cells are red, HA-binding cells are green, and costaining is yellow.
Discussion
Many factors contribute to elevated levels of GAGs. GAGs exist excessively in connective tissue and synovial fluid. Inflammation, infection, or physical damage can lead to the release of soluble GAGs. An inflammatory reaction, irrespective of its cause, is followed by increased synthesis of HA in the interstitium (30). GAGs are secreted during the activation of various cells, for example, endothelial and T cells (30, 31). Furthermore, bacterial pathogens display GAGs or GAG-like polysaccharide antigens on their surfaces, for example, group A streptococci possess an HA-rich capsule (32). Microorganisms also secret enzymes such as hyaluronidase to release GAGs from connective tissue (33, 34). Although many infectious agents can cause inflammatory arthritis, the actual antigen behind autoimmunity may be GAGs. Building on our findings, we propose in the following a pathogenetic model for the role of GAGs in connective tissue diseases. Circulating or locally released GAGs induce the clonal expansion of various GAG-binding cells, for example, T and B cells and macrophages. These cells, because of their enhanced or “matured” binding to GAGs, preferentially migrate and adhere to connective tissue where GAGs are abundant. GAGs expressed on endothelial and synovial lining cells facilitate the extravasation and adherence of GAG-binding cells from the bloodstream into GAG-rich environments, such as connective tissue and cartilage. Excessive and prolonged accumulation of these abnormal cells eventually leads to pathological symptoms, including damage of joint cartilage and bone erosion.
We usually do not generate immune responses against our own tissues, although autoreactive cells are an inevitable product of random gene rearrangement processes that yield the diverse repertoire of lymphocyte receptors. This self-tolerance is maintained by clonal deletion or silencing. Developing lymphocytes that encounter self-antigens at the site of lymphocyte development are eliminated or functionally repressed at an early stage. If a self-reactive cell encounters its self-antigen after it matures, the cell still is generally inactivated because of the added requirement of a costimulatory signal. Because GAGs can bind to many types of cell surface receptors, as well as cytokines and other soluble protein messengers (8–12), and also because polysaccharides are capable of cross-linking multiple receptors on a single cell or multiple cells (35), GAGs could act as “superantigens” and provide the necessary signals to promote the expansion of GAG-binding cells. Furthermore, irregular amounts of highly acidic and multimolecule-binding GAGs could change the microenvironment and dynamics of the immune system. GAGs may regulate hematopoietic growth factors that favor the production of GAG-binding cells. We speculate that disease development is due to an intrinsic abnormality of cell homeostasis caused by GAGs, not just a consequence of antigen recognition by GAG-binding cells in connective tissues.
Our observations have potential implications for the fundamental understanding of arthritis and possibly other rheumatic and connective tissue diseases if aberrant immune responses to GAGs are indeed involved in these conditions. GAGs are atypical carbohydrate self-antigens compared with “classic” peptide or protein antigens. How the immune system handles carbohydrates is poorly characterized at present and may be of underestimated importance. The understanding of how immunization against self-antigens like GAGs alters the immune system and causes systemic chronic disease in mice could help fill this gap in our knowledge. Self-antigenic GAGs, the correlation of in vitro cellular activity and disease prevalence, and our in vivo model could serve as a model system for the discovery and development of drugs against autoimmune connective tissue diseases. GAG binding can be used as a detection method for cells that might be correlated with or actually cause RA and other connective tissue diseases. Finally, inhibition of the abnormal growth or adhesion of immune cells reactive to GAGs may open new therapeutic avenues for the treatment of RA and related diseases.
Acknowledgments
We thank Prof. Arne Luz and Dr. Roderick Bronson for histopathological advice, Li Zhang, Amanda L. Ganong, and Yong-Hoon Choi for technical assistance, Dr. Karen Aboody for access to her fluorescence microscope, and Dr. Janina Longtine, Department of Pathology, Brigham and Women's Hospital, for human tissue specimens. M.H.R. dedicates this work to the memory of his late father, Dr. Michael A. Roehrl.
Abbreviations
RA, rheumatoid arthritis
CSA, chondroitin sulfate A
CSB, chondroitin sulfate B
CSC, chondroitin sulfate C
GAG, glycosaminoglycan
HA, hyaluronic acid
HP, heparin
References
- 1.Reichlin M. (2001) in Arthritis and Allied Conditions, ed. Koopman, W. (Lippincott, Philadelphia), pp. 1445–1479.
- 2.Centers for Disease Control (2001) Morbid. Mortal. Wkly. Rep. 50, 120-125. [Google Scholar]
- 3.Hale L. & Haynes, B. (2001) in Arthritis and Allied Conditions, ed. Koopman, W. (Lippincott, Philadelphia), pp. 1103–1127.
- 4.Chakrabarti B. & Park, J. W. (1980) CRC Crit. Rev. Biochem. 8, 225-313. [DOI] [PubMed] [Google Scholar]
- 5.Couchman J. (2001) in Arthritis and Allied Conditions, ed. Koopman, W. (Lippincott, Philadelphia), pp. 209–225.
- 6.Engstrom-Laurent A. & Hallgren, R. (1985) Ann. Rheum. Dis. 44, 83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engstrom-Laurent A., Feltelius, N., Hallgren, R. & Wasteson, A. (1985) Ann. Rheum. Dis. 44, 614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day A. J. & Sheehan, J. K. (2001) Curr. Opin. Struct. Biol. 11, 617-622. [DOI] [PubMed] [Google Scholar]
- 9.Capila I. & Linhardt, R. (2002) Angew. Chem. Int. Ed. Engl. 41, 390-412. [DOI] [PubMed] [Google Scholar]
- 10.Kuschert G. S. V., Coulin, F., Power, C. A., Proudfoot, A. E. I., Hubbard, R. E., Hoogewerf, A. J. & Wells, T. N. C. (1999) Biochemistry 38, 12959-12968. [DOI] [PubMed] [Google Scholar]
- 11.Fujii K., Tanaka, Y., Hubscher, S., Saito, K., Ota, T. & Eto, S. (1999) J. Immunol. 162, 2391-2398. [PubMed] [Google Scholar]
- 12.Tanaka Y., Fujii, K., Hubscher, S., Aso, M., Takazawa, A., Saito, K., Ota, T. & Eto, S. (1998) Arthritis Rheum. 41, 1365-1377. [DOI] [PubMed] [Google Scholar]
- 13.Bradford M. (1976) Anal. Biochem. 72, 248-254. [DOI] [PubMed] [Google Scholar]
- 14.De Belder A. N. & Ove Wik, K. (1975) Carbohydr. Res. 44, 251-257. [DOI] [PubMed] [Google Scholar]
- 15.Hirose J., Kawashima, H., Yoshie, O., Tashiro, K. & Miyasaka, M. (2001) J. Biol. Chem. 276, 5228-5234. [DOI] [PubMed] [Google Scholar]
- 16.Tzianabos A. O., Finberg, R. W., Wang, Y., Chan, M., Onderdonk, A. B., Jennings, H. J. & Kasper, D. L. (2000) J. Biol. Chem. 275, 6733-6740. [DOI] [PubMed] [Google Scholar]
- 17.Wrenshall L. E., Stevens, R. B., Cerra, F. B. & Platt, J. L. (1999) J. Leukocyte Biol. 66, 391-400. [DOI] [PubMed] [Google Scholar]
- 18.Goldblatt D. (1998) J. Med. Microbiol. 47, 563-567. [DOI] [PubMed] [Google Scholar]
- 19.Bridges S. (2001) in Arthritis and Allied Conditions, ed. Koopman, W. (Lippincott, Philadelphia), pp. 1223–1244.
- 20.Sugawara I. & Ishizaka, S. (1982) Cell. Immunol. 74, 162-171. [DOI] [PubMed] [Google Scholar]
- 21.Rachmilewitz J. & Tykocinski, M. L. (1998) Blood 92, 223-229. [PubMed] [Google Scholar]
- 22.Xia C.-Q. & Kao, K.-J. (2002) J. Immunol. 168, 1131-1138. [DOI] [PubMed] [Google Scholar]
- 23.Termeer C. C., Hennies, J., Voith, U., Ahrens, T., Weiss, J. M., Prehm, P. & Simon, J. C. (2000) J. Immunol. 165, 1863-1870. [DOI] [PubMed] [Google Scholar]
- 24.Teder P., Vandivier, R. W., Jiang, D., Liang, J., Cohn, L., Pure, E., Henson, P. M. & Noble, P. W. (2002) Science 296, 155-158. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury J. (2002) Lancet 359, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Weyand C. M. (2000) Rheumatology 39 Suppl. 1, 3-8. [DOI] [PubMed] [Google Scholar]
- 27.Firestein G. S. & Zvaifler, N. J. (2002) Arthritis Rheum. 46, 298-308. [DOI] [PubMed] [Google Scholar]
- 28.Wagner U. G., Koetz, K., Weyand, C. M. & Goronzy, J. J. (1998) Proc. Natl. Acad. Sci. USA 95, 14447-14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda Y., Masuko, K., Nakai, Y., Kato, T., Hasanuma, T., Yoshino, S. I., Mizushima, Y., Nishioka, K. & Yamamoto, K. (1996) Arthritis Rheum. 39, 446-453. [DOI] [PubMed] [Google Scholar]
- 30.Gerdin B. & Hallgren, R. (1997) J. Intern. Med. 242, 49-55. [DOI] [PubMed] [Google Scholar]
- 31.Fraser J. R., Laurent, T. C. & Laurent, U. B. (1997) J. Intern. Med. 242, 27-33. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham M. W. (2000) Clin. Microbiol. Rev. 13, 470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menzel E. J. & Farr, C. (1998) Cancer Lett. 131, 3-11. [DOI] [PubMed] [Google Scholar]
- 34.Gillespie S. H. & Balakrishnan, I. (2000) J. Med. Microbiol. 49, 1057-1067. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Kalka-Moll, K. M., Roehrl, M. H. & Kasper, D. L. (2000) Proc. Natl. Acad. Sci. USA 97, 13478-13483. [DOI] [PMC free article] [PubMed] [Google Scholar]