Abstract
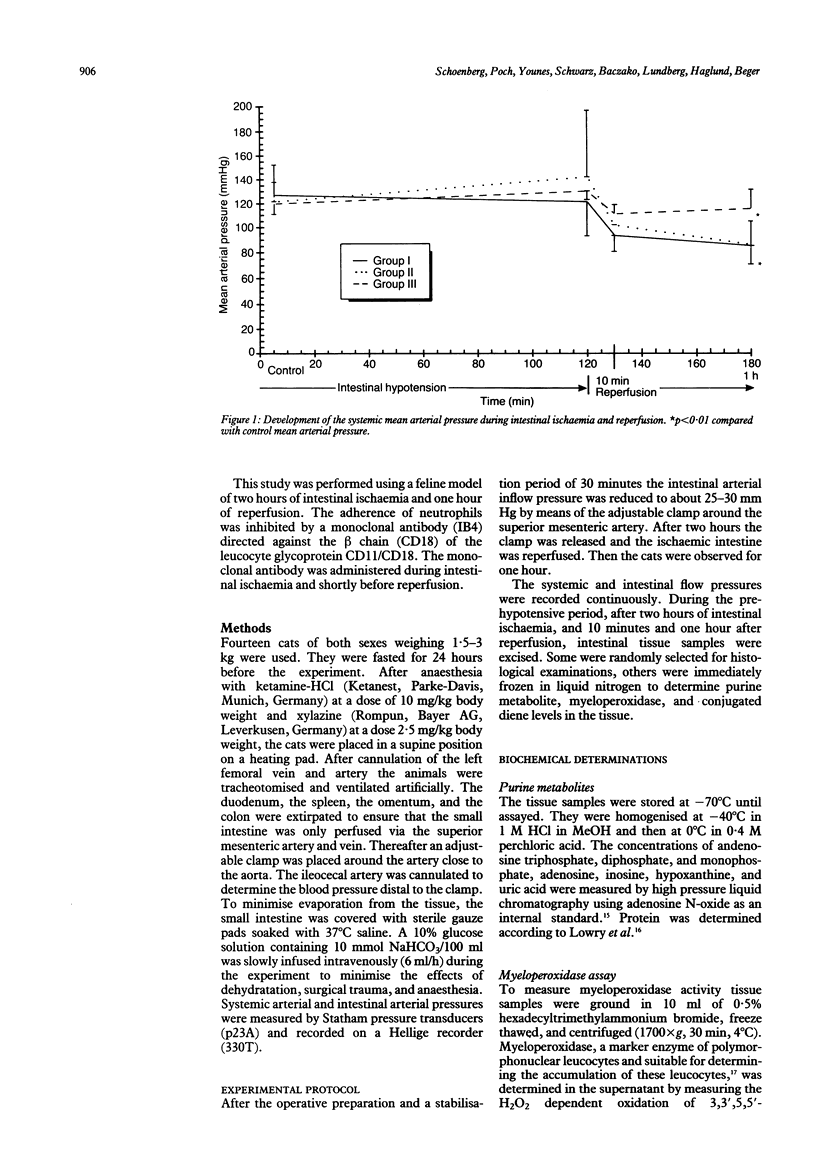
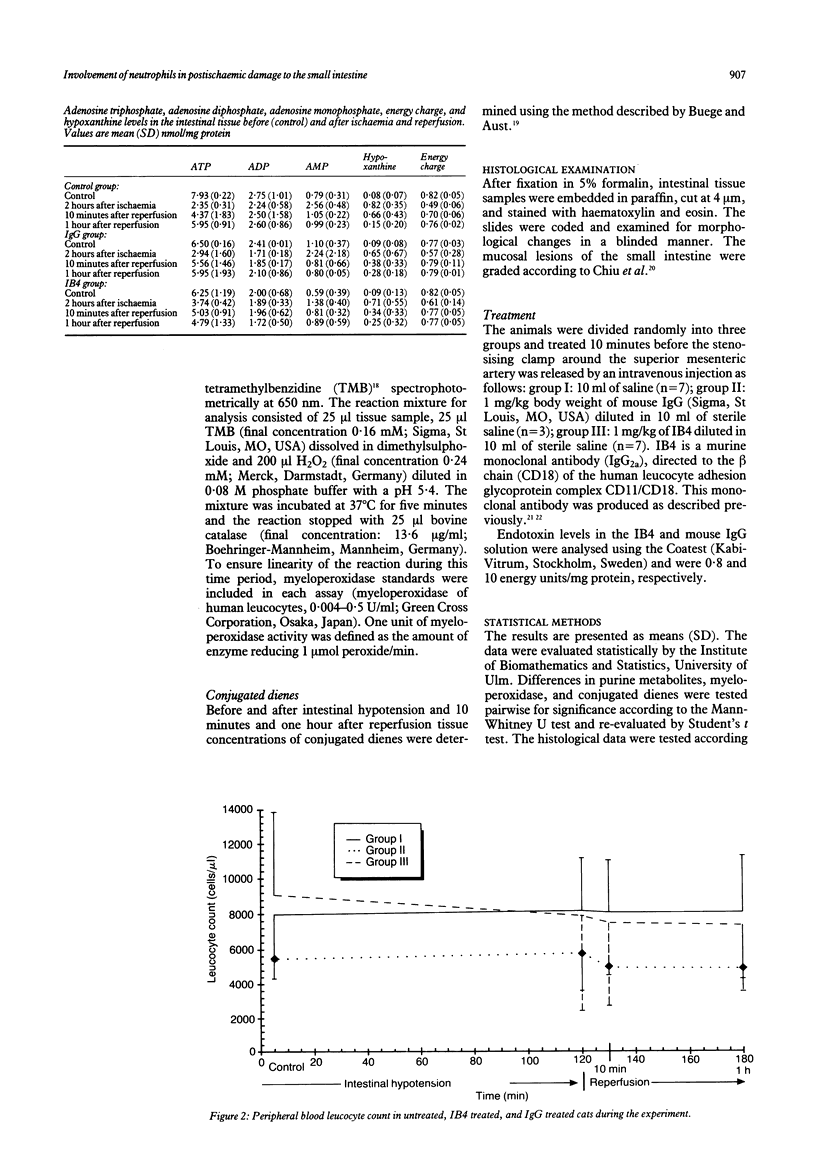
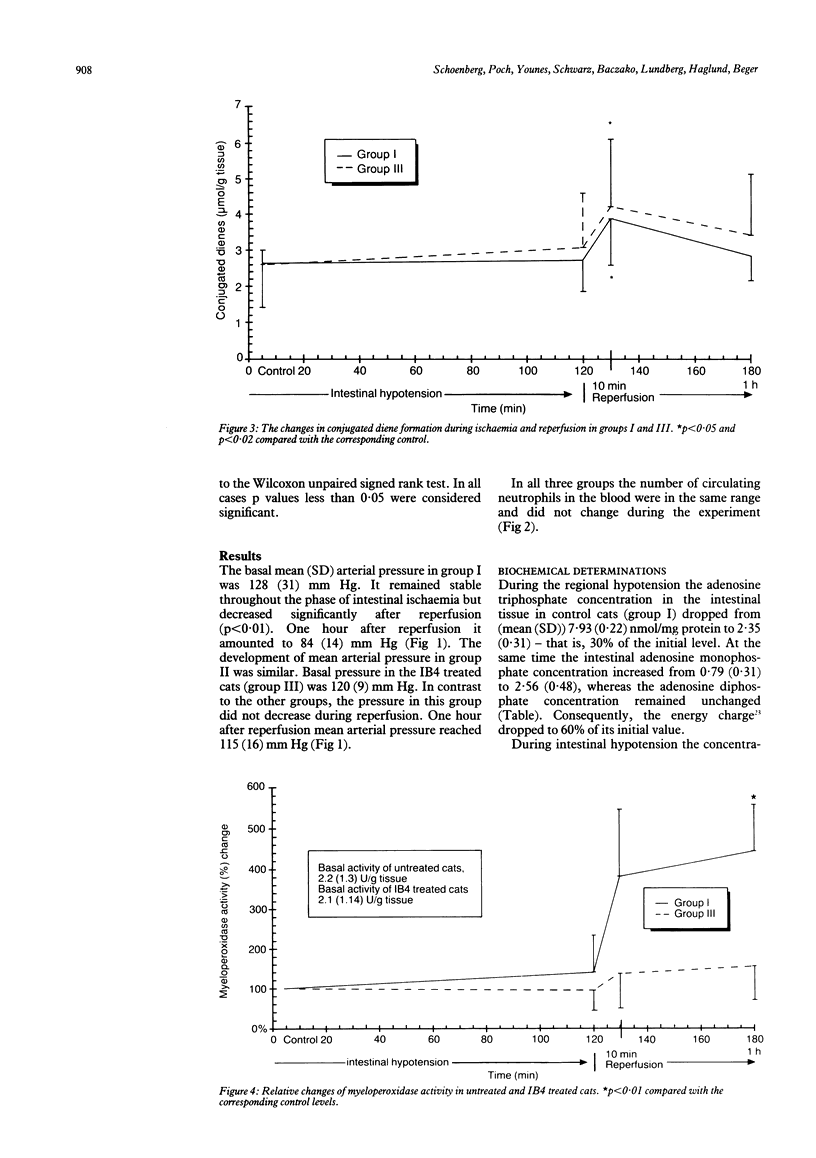
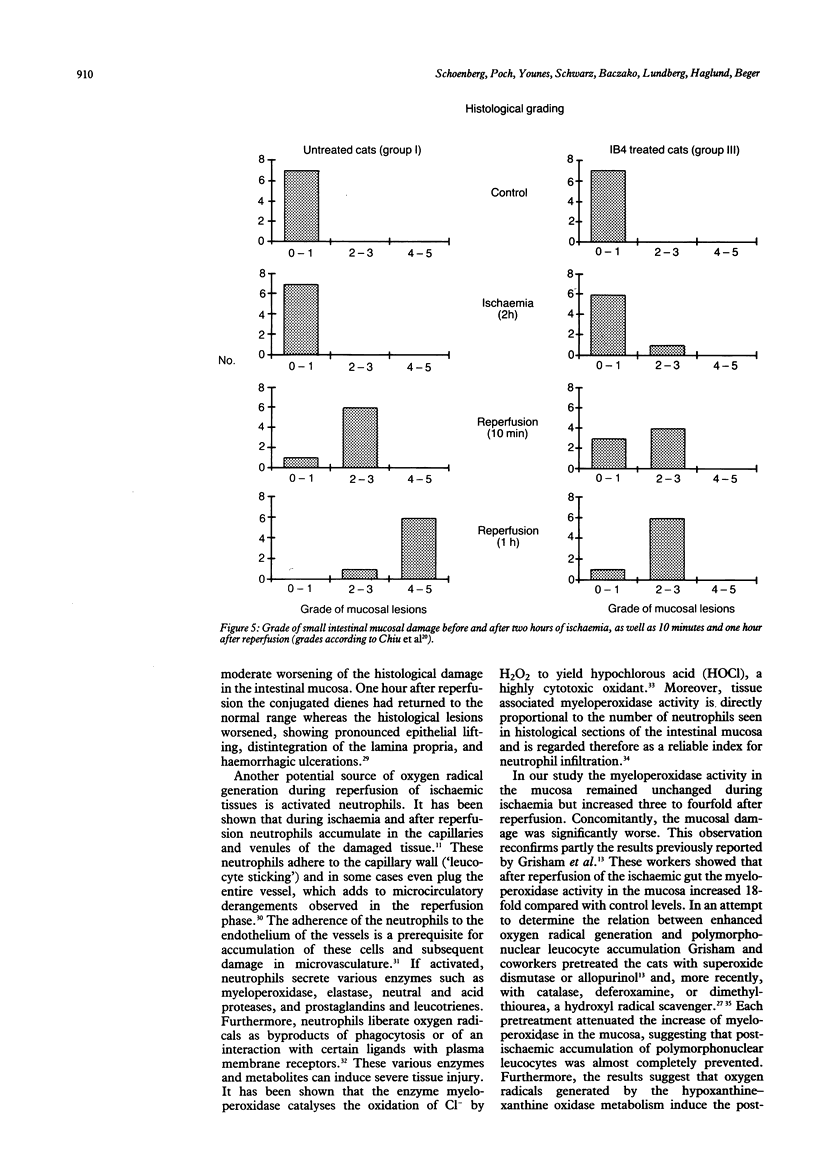
Haemorrhagic mucosal lesions are produced during intestinal ischaemia and after reperfusion probably mediated by oxygen radicals. Oxygen radicals react with cell membrane lipids and induce cell damage by peroxidation and induce accumulation of polymorphonuclear leucocytes in the tissue. The aim of the study was to elucidate the involvement of polymorphonuclear leucocytes in post-ischaemic intestinal damage. Intestinal ischaemia was induced in cats by partial occlusion of the superior mesenteric artery. Samples from the small intestine were excised before and at the end of the two hour hypotensive period as well as 10 minutes and 60 minutes after reperfusion. Conjugated dienes, myeloperoxidase, and the purine metabolites were determined in the samples. The tissue was also examined histologically. Seven cats were treated before reperfusion with a monoclonal antibody (IB4) which inhibits leucocyte adherence to endothelial cells and its subsequent activation. After reperfusion myeloperoxidase activity increased and the ischaemic mucosal lesions worsened significantly. IB4 treatment prevented an increase in post-hypotensive myeloperoxidase activity and attenuated the normally observed severe mucosal lesions. We conclude that the severe post-ischaemic lesions are induced by polymorphonuclear leucocytes. Such mucosal injury may be appreciably reduced by blocking leucocyte adherence with IB4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Björk J., Arfors K. E. Oxygen free radicals and leukotriene B4 induced increase in vascular leakage is mediated by polymorphonuclear leukocytes. Agents Actions Suppl. 1982;11:63–72. [PubMed] [Google Scholar]
- Chiu C. J., McArdle A. H., Brown R., Scott H. J., Gurd F. N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970 Oct;101(4):478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- Del Maestro R. F., Björk J., Arfors K. E. Increase in microvascular permeability induced by enzymatically generated free radicals. II. Role of superoxide anion radical, hydrogen peroxide, and hydroxyl radical. Microvasc Res. 1981 Nov;22(3):255–270. doi: 10.1016/0026-2862(81)90096-0. [DOI] [PubMed] [Google Scholar]
- Granger D. N., McCord J. M., Parks D. A., Hollwarth M. E. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986 Jan;90(1):80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- Granger D. N., McCord J. M., Parks D. A., Hollwarth M. E. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986 Jan;90(1):80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- Granger D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988 Dec;255(6 Pt 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Gregory J. E., Proske U. Motor unit contractions initiating impulses in a tendon organ in the cat. J Physiol. 1981;313:251–262. doi: 10.1113/jphysiol.1981.sp013662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Haglund U., Abe T., Ahrén C., Braide I., Lundgren O. The intestinal mucosal lesions in shock. II. The relationship between the mucosal lesions and the cardiovascular derangement following regional shock. Eur Surg Res. 1976;8(5):448–460. doi: 10.1159/000127889. [DOI] [PubMed] [Google Scholar]
- Haglund U., Bulkley G. B., Granger D. N. On the pathophysiology of intestinal ischemic injury. Clinical review. Acta Chir Scand. 1987;153(5-6):321–324. [PubMed] [Google Scholar]
- Haglund U., Hultén L., Ahren C., Lundgren O. Mucosal lesions in the human small intestine in shock. Gut. 1975 Dec;16(12):979–984. doi: 10.1136/gut.16.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Platelet-activating factor-induced microvascular dysfunction: role of adherent leukocytes. Am J Physiol. 1990 Jan;258(1 Pt 1):G158–G163. doi: 10.1152/ajpgi.1990.258.1.G158. [DOI] [PubMed] [Google Scholar]
- LILLEHEI R. C., LONGERBEAM J. K., BLOCH J. H., MANAX W. G. THE MODERN TREATMENT OF SHOCK BASED ON PHYSIOLOGIC PRINCIPLES. Clin Pharmacol Ther. 1964 Jan-Feb;5:63–101. doi: 10.1002/cpt19645163. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundberg C., Arfors K. E. Polymorphonuclear leukocyte accumulation in inflammatory dermal sites as measured by 51Cr-labeled cells and myeloperoxidase. Inflammation. 1983 Sep;7(3):247–255. doi: 10.1007/BF00917262. [DOI] [PubMed] [Google Scholar]
- Lundberg C., Wright S. D. Relation of the CD11/CD18 family of leukocyte antigens to the transient neutropenia caused by chemoattractants. Blood. 1990 Sep 15;76(6):1240–1245. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc. 1987 May 15;46(7):2397–2401. [PubMed] [Google Scholar]
- Schoenberg M. H., Fredholm B. B., Haglund U., Jung H., Sellin D., Younes M., Schildberg F. W. Studies on the oxygen radical mechanism involved in the small intestinal reperfusion damage. Acta Physiol Scand. 1985 Aug;124(4):581–589. doi: 10.1111/j.1748-1716.1985.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. H., Muhl E., Sellin D., Younes M., Schildberg F. W., Haglund U. Posthypotensive generation of superoxide free radicals--possible role in the pathogenesis of the intestinal mucosal damage. Acta Chir Scand. 1984;150(4):301–309. [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983 Jul 15;132(2):345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Inauen W., Kvietys P. R., Grisham M. B., Meininger C., Schelling M. E., Granger H. J., Granger D. N. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol. 1989 Nov;257(5 Pt 2):H1740–H1745. doi: 10.1152/ajpheart.1989.257.5.H1740. [DOI] [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988 Mar;81(3):939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M., Schoenberg M. H., Jung H., Fredholm B. B., Haglund U., Schildberg F. W. Oxidative tissue damage following regional intestinal ischemia and reperfusion in the cat. Res Exp Med (Berl) 1984;184(4):259–264. doi: 10.1007/BF01852385. [DOI] [PubMed] [Google Scholar]


